Healthy Individuals With Elevated LDL-C: Does Treatment Help?
Introduction
The intent of this post is to provide additional insight and perspective regarding individuals who are metabolically healthy but have an elevated LDL-C and/or Apolipoprotein-B (ApoB) of unclear significance. Importantly, this is not a topic of settled debate, but rather, an area where we will benefit from ongoing research. Over the past five years, expert consensus on subject matter has changed rather significantly and it would be naïve to assume that we have it all figured out at this time. In fact, that is a major part of the problem. We do not have it all figured out and the lack of certainty contributes to the challenge. Specifically, it is recognized that LDL-C and ApoB containing lipoproteins contribute to atherosclerosis in patients with established ASCVD risk factors. Does this same level of risk apply to healthy individuals without traditional cardiovascular risk factors? Do healthy individuals benefit from efforts to reduce LDL-C/ApoB? While this remains an area of active debate and ongoing research, fortunately, there are several diagnostic tools and treatments available to alleviate some of this burden of uncertainty.
A question that troubles many… if you are metabolically healthy, to what extent will lipid lowering benefit improve healthspan and lifespan? In an attempt to distinguish myself from others who advocate for the aggressive lowering of LDL-C/ApoB in healthy individuals, I will provide a thorough examination of the existing clinical evidence. While many will be quick to acknowledge the resounding success of lipid lowering therapy and its ability to reduce ASCVD, it is necessary to consider that the overwhelming majority of this success has been achieved in patients who are at high-risk of cardiovascular disease, and the applicability of this data to a healthy individual is unclear. The content of this post will likely be thought-provoking and may challenge your currently held beliefs. Like always, my goal is not to be controversial or sensational, but rather, to be informative, scientific, and objective. In the process of maintaining an open-mind, we can all learn from one another and become more informed.
As we will investigate in greater detail below, in healthy individuals without pre-existing cardiovascular disease, lipid lowering therapy has not been shown to consistently prolong lifespan. Meanwhile, there is stronger evidence to suggest that lipid lowering therapy may reduce the occurrence of cardiovascular events, such as heart attack and stroke, which can be characterized as an improvement in healthspan. The failure to consistently achieve a mortality benefit in low-risk patients may either be due to (1) the limited duration of clinical trials compared to the natural time course of atherosclerosis, for which longer trials may demonstrate a mortality benefit, or (2) that lipid lowering therapy does not consistently prolong lifespan in patients at low-risk of ASCVD. Importantly, however, lipid lowering therapies are generally safe and well-tolerated, which fuels the consideration to initiate lipid lowering therapy in low-risk individuals. In other words, if it’s safe and potentially effective, many would argue that this possibility is worth pursuing, especially when considering the cumulative burden of cardiovascular disease. Importantly, there are also many health-conscious individuals perceived as healthy or at “low cardiovascular risk,” when in fact, unidentified ASCVD risk factors may be present (Tables 9-10). Therefore, a thorough medical evaluation is advisable for individuals with elevated levels of LDL-C/ApoB.
Ultimately, I do not believe there is one obvious or “correct” recommendation for the decision-making process of healthy individuals with elevated levels of LDL-C/ApoB. Rather, this post will seek to portray multiple reasonable perspectives, which should ultimately be guided by the individual’s values and priorities, with additional assistance from their licensed healthcare professional. Furthermore, each person’s definition of risk will vary, highlighting the personal nature of this subject matter.
General Disclaimer
This content is for general educational purposes only and does not represent medical advice or the practice of medicine. Furthermore, no patient relationship is formed. Please discuss with your healthcare provider before making any dietary, lifestyle, or pharmacotherapy changes.
Notify Me of New Content
Provide your email address to receive notifications of new blog posts and podcast episodes.
Content Summary
A Clinical Conundrum: Elevated LDL-C/ApoB In The Metabolically Healthy
Cardiovascular disease is the number one cause of death among adult men and women throughout the world. It is the second leading cause of death among young adults ages 45 to 64 years.1 Meanwhile, a key risk factor of cardiovascular disease is an elevated level of low-density lipoprotein (LDL), as well as Apolipoprotein-B (ApoB), which has emerged as a stronger predictor of ASCVD than LDL-C. As a result, a significant priority among healthcare professionals and health-conscious individuals has been the aggressive reduction of LDL-C/ApoB levels using a combination of dietary modification, physical exercise, and prescription medications.
A notable challenge within this subject area is the appropriate risk stratification of elevated LDL-C/ApoB levels in healthy individuals. Specifically, how worried should this population be regarding elevated LDL-C/ApoB in the absence of traditional ASCVD risk factors? In addition to optimal dietary and lifestyle modifications, is lipid lowering beneficial or advisable? These are important questions, for which no clear answer has been agreed upon. While a significant amount of scientific evidence exists regarding lipid lowering therapy for the prevention of ASCVD in individuals without ASCVD (primary prevention), the greatest challenge in the interpretation of this evidence is that high-risk patients are disproportionately represented in these trials, with a paucity of research evaluating long-term outcomes in metabolically healthy individuals. This complicates the applicability and external validity of the data interpretation.
Needless to say, further research will help to clarify answers to these questions. Until then, a framework is described below to optimize this decision-making process, which relies heavily on the individual’s personal values, healthcare priorities, and perceived definition of risk.
Perspective 1: Early Implementation of Lipid-Lowering Therapy
Today, there are many effective medications available to effectively lower LDL-C and ApoB. These therapies have established long-term safety and are generally well-tolerated. In the event that a medication side-effect occurs, the side-effects are generally reversible. Meanwhile, multiple alternative therapies remain available. Therefore, with patients with an elevated LDL-C/ApoB without traditional risk factors, it is reasonable to consider the initiation of lipid lowering therapy for the sake of reducing the cumulative exposure of LDL/ApoB, and thus, the likelihood of ASCVD. Ultimately, the risk of developing atherosclerosis appears greater than the risk of medication-induced side-effects.
Perspective 2: Regular and Routine Surveillance of ASCVD
While levels of LDL-C/ApoB can be easily reduced with prescription medications, the overall benefit is likely relatively modest and perhaps negligible in many healthy individuals. In place of early and aggressive lipid lowering therapy for low-risk patients, there are several non-invasive tools that can be safely implemented to detect ASCVD early. This includes coronary artery calcium (CAC) scoring or CT Coronary Angiography (CTCA). These imaging techniques can be obtained quickly, affordability, and safely. If coronary calcification/atherosclerosis is present, lipid lowering therapy is then initiated. If there is no detectable coronary calcification or atherosclerosis, repeat CT imaging surveillance should be considered. Ultimately, it is felt that the risk of healthy individuals developing significant atherosclerosis while receiving proactive surveillance is relatively low. Separately, if a patient develops traditional ASCVD risk factor(s), lipid lowering therapy may be initiated in the absence of detected coronary atherosclerosis/calcification.
Importantly, Perspective 2 is influenced by the recognition that medication-induced side-effects do occur, for which the most common statin-associated side effects are (1) blood glucose dysregulation and (2) elevated liver enzymes . Both of these adverse outcomes are regularly observed and their clinical significance remains unclear. Fortunately, both can be monitored and the effects are generally reversible with discontinuation of the causative medicine. Aside from statin therapy, Ezetimibe is generally safe and effective. Meanwhile, the affordability, accessibility, and prescriber familiarity with newer classes of lipid lowering therapy remain a barrier, for which the use of statin therapy remains the most widely used class of medicine.
Limitations of Perspective 2
The most apparent limitation of Perspective 2 is that the proactive approach to detect early coronary calcification and/or atherosclerosis is in fact a reactive approach. Specifically, a decision to initiate lipid lowering therapy based upon Perspective 2 would not be triggered until the presence of ASCVD has developed, which obviously, is undesirable. With that said, proactive surveillance would likely detect atherosclerosis at an early and clinically insignificant stage.
Balancing Perspectives
As previously stated, it becomes clear that an individual’s personal values and healthcare goals become highly relevant in the decision-making process. Furthermore, one’s definition of risk is also highly relevant… is the risk of atherosclerosis more concerning than the risk of lifelong prescription medications, their potential side-effects, and the possibility that there may be no benefit to the individual? Meanwhile, with aging and the accumulation of ASCVD risk factors, the decision to initiate lipid lowering may become more clear.
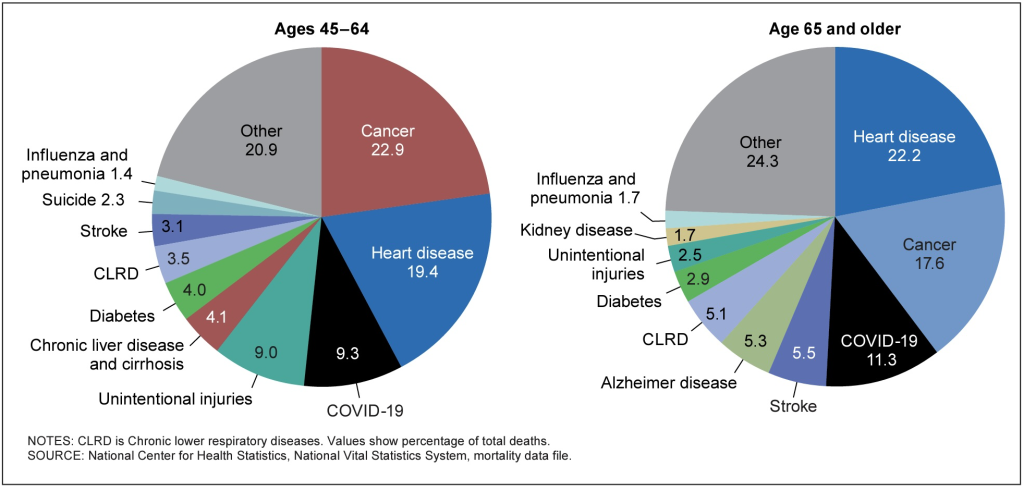
Figure 1. Distribution of the 10 leading causes of death, United States, 2020
Note: It is necessary to acknowledge the relatively low stated impact of diabetes on total mortality in the United States. The reader should be aware that diabetes and insulin resistance contribute to the risk and progression of heart disease, stroke, certain cancers, dementia, kidney disease, fatty liver disease and non-alcoholic cirrhosis, and severe respiratory illness and death from COVID-19, influenza, and pneumonia.
Available Tools and Shortcomings
Current medical guidelines utilize ASCVD risk calculators to assist with clinical decision making regarding lipid lowering therapy, including the Framingham Risk Score and the ASCVD Risk Estimator Plus. Unfortunately, these risk calculators consistently underestimate disease risk in younger patients.2-4 In an analysis of patients 55 years and younger diagnosed with a first-time heart attack, less than half met guideline-based eligibility for statin therapy according to the 2018 American College of Cardiology and American Heart Association (AHA/ACC) cholesterol guidelines.4 Even fewer young patients diagnosed with a heart attack met recommended lipid lowering treatment thresholds according to the 2016 U.S. Preventive Services Task Force (UPSTF) guidelines.3 Collectively, these findings demonstrate the inadequacy of traditional risk calculators for the primary prevention of ASCVD in younger adults.
Evaluating the Evidence of Lipid Lowering Therapy In Healthy Individuals
As previously stated, multiple classes of lipid lowering therapies have been established as safe and effective for the reduction of cardiovascular disease and mortality in patients without pre-existing cardiovascular disease (i.e. primary prevention). Notably, however, the overwhelming majority of patients enrolled into these clinical trials are considered high-risk individuals, for which the applicability to healthy individuals is unclear, and the interpretation of this data becomes challenging.
A meta-analysis of 29 randomized controlled trials involving a total of 80,711 healthy participants was conducted to determine the impact of statin therapy.5 The definition of low cardiovascular risk was based upon the absence of any pre-existing cardiovascular disease, and an estimated 10-year cardiovascular event risk of 20% of less. Subgroup analysis included patients treated with high-intensity and low-intensity statin therapy.
The conclusion of this meta-analysis states that “statins were found to be efficacious in preventing death and cardiovascular morbidity in people at low cardiovascular risk.” However, the analysis of the individual trials paints a less compelling conclusion, especially in those who received low-intensity statin therapy.
Among the 13 trials that evaluated the use of low-intensity statin therapy, only one trial demonstrated an improvement in lifespan. Notably, the relative risk reduction of this one study included confidence intervals with an upper limit of 0.99 (Relative Risk 0.12, 95% CI 0.02 – 0.99), implying borderline statistical significance. The pooled relative risk reduction of total mortality did not achieve statistical significance, implying that healthy individuals do not live longer while on low-intensity lipid lowering therapy (RR 0.90, 95% Confidence Interval, 0.79-1.03) (Table 1).
Regarding the six trials investigating the use of high-intensity statin therapy, again, only one trial demonstrated an improvement in lifespan. Notably, this study, JUPITER, only enrolled patients with an elevated level of high-sensitivity C-reactive protein (hs-CRP), implying abnormal levels of systemic inflammation at baseline.6 For obvious reasons, it is hard to apply these results to a truly healthy population, for which normal levels of hs-CRP are to be expected. For reasons outside of the scope of this post, there were multiple criticisms and concerns with the JUPITER trial at the time of publication, including an unusually high mortality rate in the control group.
Regardless, in this meta-analysis (not JUPITER), the combined results of the high-intensity statin trials demonstrated a statistically significant reduction in total mortality (RR 0.85, 95% CI 0.74 – 0.96). A similar mortality reduction was observed in the combined results of the low-intensity and high-intensity statin trials (RR 0.90, 95% CI 0.84 – 0.97) (Table 2).
While the pooled results of all trials demonstrated mortality benefit, again, only 2 of the 19 trials achieved a statistically significant mortality benefit within the original clinical trial.
Table 1. Risk of death from any cause in patients at low cardiovascular risk treated with low-intensity statin therapy
| Low-Intensity Statin | Total Mortality,Relative Risk | Lifespan Improved |
|---|---|---|
| EXCEL | 2.78 (95% CI, 0.85 – 9.05) | No |
| ACAPS | 0.12 (0.02 – 0.99) | Yes |
| KAPS | 0.75 (0.17 – 3.30) | No |
| WOSCOPS | 0.78 (0.61 – 1.01) | Likely |
| CAILUS | 3.06 (0.13 – 74.51) | No |
| AFCAPS/TEXCAPS | 1.04 (0.76 – 1.41) | No |
| KLIS | 0.82 (0.61 – 1.11) | No |
| ALLHAT-LLT | 0.99 ( 0.89 – 1.09) | No |
| Brickert et al. | 1.02 (0.06 – 16.35) | No |
| PHYLLIS | 3.00 (0.12 – 73.30) | No |
| PREVEND-IT | 1.08 (0.50 – 2.34) | No |
| HYRIM | 0.81 ( (0.22 – 2.97) | No |
| MEGA | 0.71 (0.51 – 1.00) | Likely |
| Combined Analysis | 0.90 (0.79 – 1.03) | No |
Note: I assume that a reduction in total mortality implies an improvement in lifespan; if you disagree with this interpretation, then replace any use of the word lifespan with total mortality. This choice of words was used for the sake of readability and applicability to this audience.
Table 2. Risk of death from any cause in patients at low cardiovascular risk treated with high-intensity statin therapy
| High-Intensity Statin | Total Mortality, Relative Risk | Lifespan Improved |
|---|---|---|
| ASCOT-LLA | 0.87 (95% CI, 0.72 – 1.06) | No |
| Homberg et al. | 1.04 (0.32 – 3.45) | No |
| METEOR | 1.21 (0.05 – 29.56) | No |
| JUPITER * | 0.80 (0.67 – 0.96) | Yes * |
| LEADe | 1.55 (0.56 – 4.31) | No |
| LORD | 0.90 (0.25 – 3.18) | No |
| Combined Analysis | 0.85 (0.74 – 0.96) | Yes |
*JUPITER enrolled patients with an elevated CRP at baseline, which contradicts the concept of evaluating the use of statin therapy in healthy individuals at “low-risk” of cardiovascular disease.
Table 3. Risk of death from any cause in patients at low cardiovascular risk treated with statin therapy, combined analysis of low and high-intensity therapy.
| Any Statin Therapy | Total Mortality, Relative Risk | Lifespan Improved |
|---|---|---|
| Overall | 0.90 (95% CI, 0.84 – 0.97) | Yes |
In this same meta-analysis of healthy patients, it can be argued that the outcomes data for healthspan was improved due to reductions in non-fatal heart attacks and non-fatal stroke. Notably, this potential benefit appears more compelling for those taking high-intensity statin therapy.
Table 4. Risk of non-fatal heart attack in patients at low cardiovascular risk
| Non-fatal Heart Attack, Relative Risk | Statistical Significance | |
|---|---|---|
| Low-Intensity Statin | 0.77 (95% CI, 0.59 – 1.00) | Borderline |
| High-Intensity Statin | 0.47 (0.34 – 0.67) | Yes |
| Combined Total | 0.64 (0.49 – 0.84) | Yes |
Table 5. Risk of non-fatal stroke in patients at low cardiovascular risk
| Non-fatal Stroke, Relative Risk | Statistical Significance | |
|---|---|---|
| Low-Intensity Statin | 0.88 (95% CI, 0.73 – 1.06) | No |
| High-Intensity Statin | 0.51 (0.33 – 0.79) | Yes |
| Combined Total | 0.81 (0.68 – 0.96) | Yes |
Evaluating the Evidence of Lipid Lowering Therapy In Healthy Individuals, Part 2.
In a separate meta-analysis, data was evaluated from 22 randomized clinical trials that tested low-intensity statin therapy in patients that were separated into five categories based upon their estimated 5-year major vascular event risk: <5%, ≥5% to <10%, ≥10% to <20%, ≥20% to <30%, ≥30%.7 Of note, five studies in this meta-analysis were also evaluated in the previously discussed meta-analysis. While this study sought to evaluate the risk reduction of lipid lowering therapy in patients with “low risk of vascular disease,” the definition of this low-risk matters. Among all categories of patients, 21% of study participants had diabetes, 39% had established coronary artery disease, and 20% had other types of vascular disease, including peripheral vascular disease. It is difficult to accept these as “low risk” patients given the established presence of vascular disease and/or the presence of diabetes mellitus, which are both very high-risk conditions. Upon taking a closer look, in patients with a 5-year major event risk of 10-20%, 24% had diabetes, 33% had established coronary artery disease, and 22% had other types of vascular disease. Even among patients with an estimated 5-10% 5-year major event risk, 18% of patients had diabetes, for which it is expected that the utilization of lipid lowering therapy in patients with established cardiovascular disease and/or diabetes will experience benefit. Therefore, once again, the relevance of these results are difficult to apply to healthy individuals.
Because of the relatively low rates of pre-existing cardiovascular disease and diabetes in the two groups of patients with a 5-year major event risk of <5% and 5-10%, it is worthwhile and relevant to evaluate these results. In patients without pre-existing vascular disease, serious cardiac events and stroke were reduced in both the <5% and 5-10% risk categories (Table 6). Notably, cardiovascular mortality and non-cardiovascular mortality was not improved in either of the <5% and 5-10% risk categories, implying that lipid lowering therapy achieves a reduction in cardiovascular events (i.e. improvement in healthspan), without compelling evidence that lifespan is improved. This is largely consistent with the previously discussed meta-analysis.
Table 6. Major vascular event reduction in low-risk patients receiving statin
| Estimated Risk of Major Vascular Event | Major Vascular Event, Relative Risk | Statistically Significant |
|---|---|---|
| < 5% | 0.61 (95% CI 0.45 – 0.81) | Yes |
| 5 to 10% | 0.66 (0.57 – 0.77) | Yes |
Table 7. Vascular and non-vascular death in low-risk patients receiving statin therapy
| Estimated Risk of Major Vascular Event | Vascular Death, Relative Risk | Non-vascular Death, Risk Reduction | Statistically Significant |
|---|---|---|---|
| < 5% | 0.80 (0.43 – 1.47) | 1.13 (0.76 – 1.69) | No |
| 5 to 10% | 0.75 (0.55 – 1.04) | 0.87 (0.67 – 1.11) | No |
Section Summary
Data from randomized clinical trials in healthy patients is limited. The existing evidence for healthy patients treated with statin therapy does not consistently demonstrate an improvement in lifespan via a reduction in total mortality. Rather, the overwhelming majority of studies failed to demonstrate a mortality benefit. When pooling results from all available trials, a mortality benefit emerges, however, this should be interpreted with caution. Meanwhile, there appears to be stronger evidence to suggest that statin therapy may reduce heart attack and stroke. Additionally, it is possible that the typical trial duration is not long enough to capture or demonstrate mortality benefits due to the natural time course of ASCVD spanning years or decades.
Outcomes of Lipid Lowering Therapy Stratified By Baseline LDL-C Levels
To further refine the question of who may benefit from lipid lowering therapy, the next section of this post will investigate cardiovascular outcomes in patients based upon their baseline LDL-C levels. Again, these trials enrolled primarily moderate and high-risk individuals, for which the applicability to healthy individuals is limited. Nevertheless, the data is interesting to review and contemplate.
In this meta-analysis of 34 randomized clinical trials that included 270,288 participants, including those with high-risk of cardiovascular disease, the greatest benefit from lipid lowering therapy occurred in patients with LDL-C levels of 100 mg/dL or greater, with increasing benefit achieved in the individuals with the highest baseline LDL-C level.8 Therefore, it can be safely concluded that lipid lowering therapy in healthy individuals with an LDL-C below 100 mg/dL are unlikely to derive cardiovascular benefit. Above 100 mg/dL, greater benefit was achieved with increasing baseline levels of LDL-C (Table 8). Therefore, it can be inferred that baseline LDL-C levels should factor into the decision of +/- lipid lowering therapy, for which an individual with a higher baseline LDL-C may have a higher likelihood of benefit. Again, this is an intuitive and obvious point that the reader has likely already assumed to be true. Meanwhile, this data is not directly applicable to a healthy or low-risk population, which should also be considered.
Table 8. All-cause Mortality Stratified by Baseline LDL-C In High, Medium and Low Risk Patients without Pre-existing Cardiovascular Disease
| Baseline LDL-C Level | Total Mortality, Relative Risk | Lifespan Improved |
|---|---|---|
| LDL-C < 100 mg/dL | 1.00 (95% CI, 0.95 – 1.06) | No |
| LDL-C 100 – 129 mg/dL | 0.88 (0.79 – 0.98) | Yes |
| LDL-C 130 – 159 mg/dL | 0.91 (0.86 – 0.96) | Yes |
| LDL-C > 160 mg/dL | 0.72 (0.62 – 0.84) | Yes |
| Combined Total | 0.92 (0.88 – 0.96) | Yes |
Note: Again, I assume that a reduction in total mortality implies an improvement in lifespan; if you disagree with this interpretation, then replace any use of the word lifespan with total mortality.
Baseline Lipoprotein Levels In Patients With Cardiovascular Disease
To address this problem from a different perspective, it is interesting to evaluate characteristics among patients diagnosed with a heart attack. In this study, lipid panels were examined in more than 130,000 patients diagnosed with a heart attack.9 At the time of their heart attack, the average LDL-C was 104.9 mg/dL, approximately 44% of patients had an LDL-C below 100 mg/dL, and 17.6% of patients had LDL-C levels below 70 mg/dL. The average triglyceride level was 161 mg/dL and a low HDL-C was the most common lipid panel abnormality. These results highlight the fact that LDL-C is likely only one of many common risk factors that contribute to cardiovascular disease. Of note, it has been observed that LDL-C can be acutely depressed at the time of a heart attack.
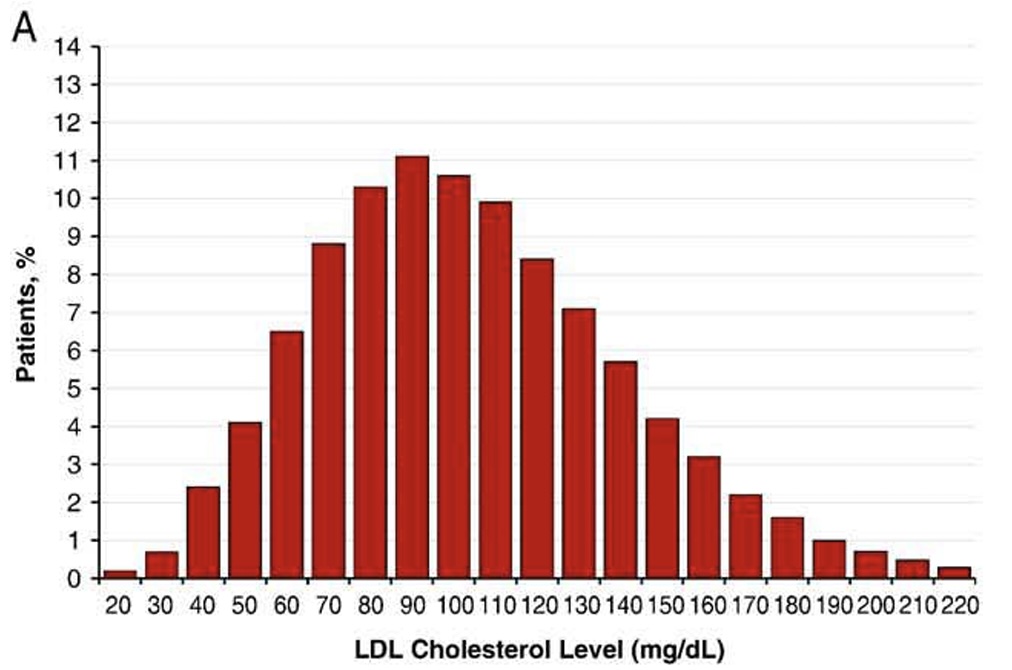
Figure 2. Distribution of LDL-C at time of hospitalization for heart attack.
Association of LDL-C and Coronary Atherosclerosis on CTCA
Meanwhile, debate remains as to whether or not an absolute LDL-C level is a useful predictive risk factor in healthy individuals. While this study does not answer that question, again, it is interesting to contemplate the data. In a study limited to 666 Japanese individuals NOT on lipid lowering therapy, they were divided into three groups based upon their LDL-C levels.10 Low LDL-C (< 70 mg/dL), Middle LDL-C (70 – 99 mg/dL), and High LDL-C (≥ 100). They then underwent coronary CT Coronary Angiography (CTCA) to characterize atherosclerosis. The amount of atherosclerosis was similar in all groups, with no predictive differences based upon LDL-C levels at the time of CTCA.
While the level of LDL-C was not associated with the presence or severity of atherosclerosis, this study is consistent with my previous post explaining that atherosclerosis still develops in patients with low levels of LDL-C, which, once again, supports the perspective that there are other non-LDL/ApoB risk factors that contribute to ASCVD.
Improving Cardiorespiratory Fitness, Lowering LDL-C/ApoB, and Your Return on Investment
For healthy individuals without any of the Traditional or Additional Risk Enhancing Features of ASCVD (Tables 9-10), evidence would suggest that the greatest opportunity to improve health is by improving cardiorespiratory fitness (CRF). Specifically, the benefit of increased physical fitness is likely greater than the benefit of lipid lowering therapy in healthy individuals. Importantly, these are not mutually exclusive strategies, for which the optimization of physical fitness, diet, and LDL-C can occur simultaneously.
The evidence to support this perspective is robust. For the sake of brevity, I will discuss data from one trial, however, there are numerous high-quality examples that demonstrate consistent findings; cardiorespiratory fitness is a stronger predictor of lifespan than all cardiometabolic risk factors, including LDL-C. Additionally, the benefit of improving cardiorespiratory fitness appears to be applicable at all fitness levels. Furthermore, it is felt that the impact of relatively small CRF improvements on mortality risk has considerable health benefits for individuals of all ages.11
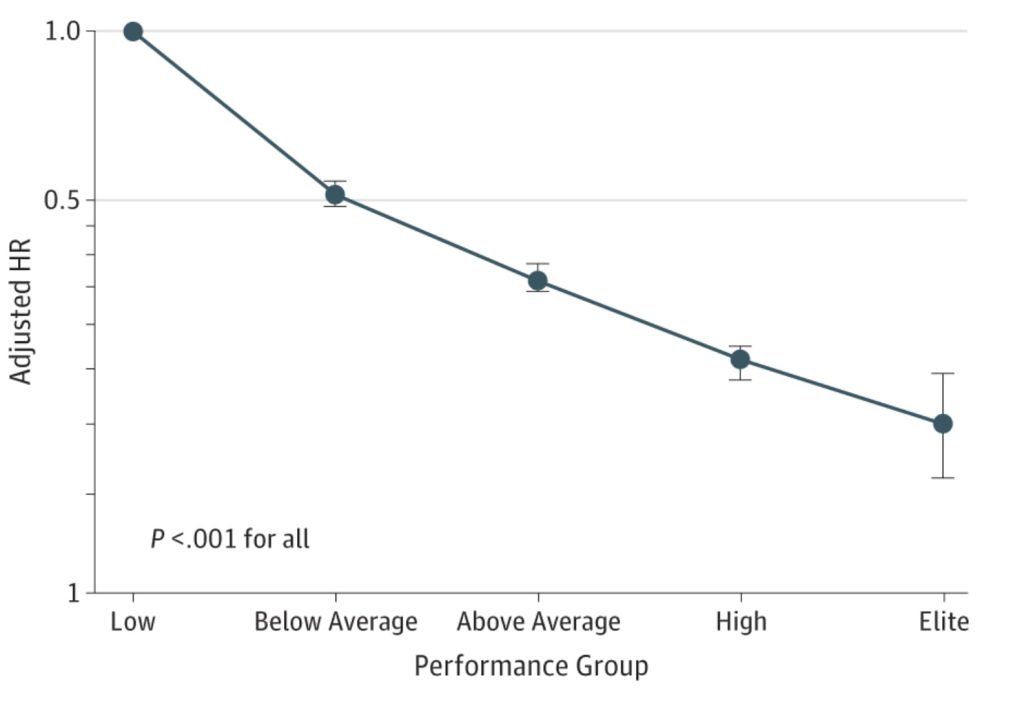
Figure 3. Adjusted hazard ratios for all-cause mortality compared with low cardiorespiratory fitness in all patients12
When To Screen for Coronary Atherosclerosis
The optimal age to begin screening for atherosclerosis via CT imaging is influenced by a variety of factors, including the individual’s metabolic health, family history, and other risk factors discussed in Tables 9-10. Among those with no ASCVD risk factors, a relevant study was published in the Journal of the American College of Cardiology (JACC).13 The specific aim of this study was to determine the ideal age to begin CAC testing in younger adults with or without risk factors present. To do so, they constructed clinical risk equations to estimate the initial conversion from CAC = 0 to CAC ≥1, which was used to guide the optimal timing of CAC testing. This study evaluated 22,346 patients aged 30 to 50, concluding that the optimal age for a first scan without risk factors was 42 years for men and 58 years for women. These ages were based upon a testing yield of 25%, implying that the equations were set to optimize the age at which 25% of individuals undergoing CAC would be identified to have a CAC > 1 (i.e. atherosclerosis present). Notably, the average age of this cohort was 43.5 years, and the total prevalence of CAC > 1 (ASCVD present) was 34%. Therefore, it can be inferred that a meaningful component of this population would have a significant delay in the detection of their atherosclerosis according to these “optimal” ages to initiate CAC in low-risk patients.
When To Initiate Lipid Lowering Therapy
As previously stated, there are multiple approaches to addressing the question, when should healthy individuals with an elevated LDL-C/Apo consider lipid lowering therapy? There is no consensus answer, and the decision itself relies heavily upon individualized decision-making, influenced by personal values, healthcare priorities, perceived risk, and guidance from their healthcare professional.
To simplify the decision-making pathway, some may elect for early lipid lowering therapy (Perspective 1), whereas others may defer lipid lowering therapy and instead implement screening for coronary atherosclerosis/calcification using CT imaging (Perspective 2). In the event that atherosclerosis/calcification is present, it would represent a clear indication for lipid lowering therapy. If no atherosclerosis/calcification is present, a decision to repeat CT imaging at 3-7 year intervals could be considered, based upon individual risk factors.14 Specifically, it has been proposed that repeat CT/CTCA can be considered at 5-7 year intervals for low-risk individuals, 3-5 years for intermediate risk individuals, and 3-years for high-risk individuals.
Importantly, there are instances when an individual may consider lipid lowering therapy in the absence of detected coronary atherosclerosis/calcification. A table is listed below to provide general perspective.
Table 9. Traditional ASCVD Risk Factors and consideration for lipid lowering therapy.
| Risk Factor | Strong Indication To Initiate Lipid Lowering Therapy | Increased Consideration For Lipid Lowering Therapy |
|---|---|---|
| Diabetes | ✓ | |
| Metabolic Syndrome | ✓ | |
| Fatty Liver | ✓ | |
| Tobacco Use | ✓ | |
| Insulin Resistance Without Diabetes | ✓ | |
| Obesity | ✓ | |
| Hypertension | ✓ | |
| Family History | ✓ | |
| Family History + Elevated Lp(a) | ✓✓ | |
| Elevated Lp(a) | ✓ | |
| Elevated Triglycerides | ✓ | |
| Elevated hs-CRP | ✓ |
Table 10. Additional Risk Enhancing Features of ASCVD
| Autoimmune and Rheumatologic Disorders | Possibly Inflammatory Bowel Disease | HIV Infection |






Dr. Forey,
Thank you for your succinct and accessible content. I hope to become a patient when you are licensed in New York.
Meanwhile I have gleaned two insights from your posts: (1) cardio respiratory fitness is the best predictor of health/longevity and (2) insulin resistance is a more potent predictor of risk than LDL(c).
My situation is complex: taking Repatha and Ezetimibe primarily because I have (had) Lp(a) of 40 mg/dl, now down to 29. All other markers are great: low trigs, very high (too high?) HDL. CAC of 0.91. Daily exercise, careful diet, low-normal BMI. But A1C has been 5.8 since 2017, maybe earlier. I take Metformin. Did genetics and discovered high risk for cardiovascular disease and stroke (specifically homozygous for C allele on RS 1333049 in the 9p21 gene). Trying to figure out what this set all means for me going forward and what to do.
Meanwhile thank you for the information. I am happy to see that cardio fitness trumps many of the other factors, and will be asking for an insulin resistance test. Hope you get your NY license soon. It is so good to connect with a physician who has a holistic view; most of the encounters I have had are with practitioners who are stuck in their silos. I have had to do my own research and advocate for myself at every step, going back to the time I was told “We don’t test for Lp(a)” and Your HDL is so good, you have nothing to worry about . . .” (My HDL is 117 — not good.)
Deborah Hall