How To Reverse Atherosclerosis: Strategies For Those With Coronary Artery Calcium (CAC)
Introduction
Atherosclerosis is the primary health abnormality underlying several common causes of cardiovascular disease, including heart attack, stroke, and cardiac valve dysfunction. More specifically, atherosclerosis is a response to injury and inflammation within the arteries supplying blood throughout the body, including the coronary arteries of the heart and carotid arteries of the brain. As the amount of atherosclerosis increases, it can contribute to atherosclerotic plaque instability, for which atherosclerotic plaque becomes susceptible to rupture and blood clot formation, resulting in the blockage of blood flow to important organs of the body, resulting in heart attack and stroke. While atherosclerosis is generally considered a chronic health abnormality, there is high-quality evidence demonstrating that atherosclerosis can be partially reversed using a variety of practical strategies, including aerobic exercise and prescription medications spanning several drug-classes. Importantly, it has been demonstrated that a 1% reduction in plaque volume is associated with an 18% reduction in cardiovascular events.1
The purpose of this article is to review the evidence demonstrating the variety of strategies capable of achieving partial reversal and regression of atherosclerosis in the human body, including aerobic exercise, the targeted lowering of atherogenic lipoproteins including LDL-C and Apolipoprotein-B (ApoB), the use of Icosapent Ethyl (Vascepa) in those with and without elevated triglycerides, blood pressure lowering agents including Angiotensin Receptor Blockers (ARBs), glucose-lowering agents such as Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitors, and medications with anti-inflammatory properties, including Colchicine. Additionally, we will review the preliminary data regarding atherosclerotic plaque regression and Glucagon-like Peptide 1 (GLP-1) Receptor Agonists. The information in this article is intended for those with a previous heart attack, stroke, carotid stenosis, aortic valve stenosis, as well as those with known atherosclerosis or calcifications visualized on coronary artery calcium scoring (CAC), coronary CT angiography (CCTA), or ultrasound imaging. Meanwhile, many of the strategies used to achieve plaque regression are relevant to those seeking to prevent the development of atherosclerosis.
Importantly, the complete reversal or elimination of established atherosclerosis is not a realistic goal with the technologies available today. Rather, while the amount of atherosclerotic plaque reversal is relatively modest, the favorable clinical impact is dramatic. Again, a 1% reduction in plaque volume is associated with an 18% reduction in major cardiovascular events.1 This is likely attributed to the fact that atherosclerotic plaque reduction is a manifestation of several favorable changes occurring simultaneously, including the improvement of risk factors that halt or significantly reduce the progression of new atherosclerosis, the stabilization of existing atherosclerotic plaque, and the visualized reduction in atherosclerotic plaque volume.
Related Podcast Episode
Disclaimer
This content is for general educational purposes only and does not represent medical advice or the practice of medicine. Furthermore, no patient relationship is formed. Please discuss with your physician before making any dietary, lifestyle, or medication changes. Additionally, I have no financial conflicts of interest or affiliations with any diagnostic testing or pharmaceutical companies mentioned.
Notify Me of New Content
Provide your email address to receive notifications of new blog posts and podcast episodes.
Content Summary
An Overview of Atherosclerosis
Atherosclerosis is the basis of the most common causes of cardiovascular disease, including heart attack, stroke, and cardiac valve dysfunction. Atherosclerosis is the biological response to chronic inflammation and injury within the arteries supplying blood throughout the human body. It is a complex process that occurs over the course of decades, and represents the manifestation of arterial wall injury, inflammation, and the accumulation of harmful lipoproteins, resulting in a pathological response of the immune system. The atherosclerotic process results in the accumulation of inflammatory cells, smooth muscle proliferation and remodeling, arterial calcification, and the formation of a fibrin cap. The progression of atherosclerosis can then result in atherosclerotic instability, plaque rupture, blood clot formation, and the obstruction of blood flow to important organs of the body, including the heart and brain, resulting in heart attack and stroke. Meanwhile, atherosclerosis and an obstruction in blood flow (ischemia) can affect essentially all arteries within the human body, with resultant damage to the eyes, kidneys, extremities, intestines, and more.
Atherosclerosis is generally considered a chronic condition, for which the overwhelming majority of affected individuals will experience the progression of atherosclerotic plaque throughout adulthood, largely attributed to the continued exposure to underlying risk factors (Tables 1-2). Meanwhile, with the emergence of advanced imaging techniques and treatment options, there is high-quality evidence demonstrating that atherosclerosis can be partially reversed using a variety of practical strategies, including aerobic exercise and multiple prescription medications spanning at least seven drug-classes. While food and nutrition represent one of the greatest modifiable risk factors for the development and progression of atherosclerosis (and many other preventable medical illnesses), there is no high-quality evidence demonstrating dietary modification and a causal reduction of atherosclerotic plaque volume. Importantly, there is high-quality evidence regarding dietary intervention and the reduction of cardiovascular death and disease,24 however, these trials did not evaluate atherosclerotic plaque volume and will not be addressed in this article.
Table 1. The Association of Common Medical Illnesses and Cardiovascular Disease25
| ASCVD Before Age 55 Adjusted Hazard Ratio | ASCVD Before Ages 65 – 75 Adjusted Hazard Ratio | |
| Diabetes | 10.71 | 3.47 |
| Metabolic Syndrome | 6.09 | 1.79 |
| Hypertension | 4.58 | 1.64 |
| Obesity, BMI > 30 kg/m2 | 4.33 | 1.32 |
Table 2. The Association of Cardiometabolic Risk Factors and Cardiovascular Disease25
| Risk Factor, per SD Increment | Age of Onset < 55 Years Adjusted Hazard Ratio | Age of Onset 65 – 75 Years Adjusted Hazard Ratio |
| Insulin Resistance, LPIR Score | 6.40 | 2.09 |
| Systolic Blood Pressure | 2.24 | 1.48 |
| Triglycerides | 2.14 | 1.61 |
| Apolipoprotein B (ApoB) | 1.89 | 1.52 |
| C-Reactive Protein (CRP) | 1.76 | 1.62 |
| Body Mass Index (BMI) | 1.47 | 1.33 |
| LDL Cholesterol | 1.38 | 1.24 |
| Hemoglobin A1c | 1.38 | 1.24 |
| Lipoprotein(a) | 1.22 | 1.11 |
The Association of Atherosclerosis and Cardiovascular Disease
As a result of non-invasive screening and imaging technologies, many individuals identify the presence of atherosclerosis prior to the onset of a heart attack, stroke, or other complication of atherosclerosis. As a result, the early identification of atherosclerosis can serve as important motivation and justification to aggressively modify existing cardiovascular risk factors for the sake of preventing the progression of atherosclerosis, or as this article will discuss, to induce plaque regression and a reduction in plaque volume.
Importantly, the amount of atherosclerosis present corresponds with the likelihood of experiencing a complication of cardiovascular disease. For example, using data from MESA, a large prospective cohort study of more than 6,800 adults comprised of diverse ages and ethnicities, there was a strong linear relationship between the amount of atherosclerosis detected on coronary artery calcium screening (CAC) and the likelihood of a cardiovascular event spanning a 10-year period.2 Specifically, among all age groups and ethnic backgrounds, a CAC score of 0 was associated with a 10-year cardiovascular event rate of 3.2%, while for those with a CAC score of 300+ had a 10-year cardiovascular event rate of 17.5% (Table 3). For each doubling of CAC, it was estimated that there was a 14% relative increment in cardiovascular disease risk. This association was not significantly affected by age, sex, race, or ethnicity.2
Table 3. 10-Year Cardiovascular Event Rate by Categories of Coronary Artery Calcium (CAC)2
| CAC = 0 | CAC 1 to 100 | CAC 101 to 300 | CAC 300+ | |
| 10-Year Cardiovascular Event Rate | 3.2% | 8.0% | 13.4% | 17.5% |
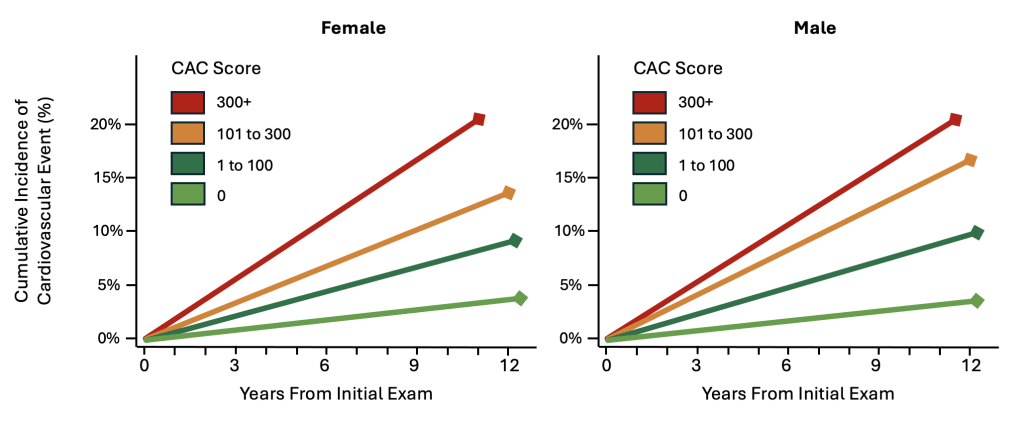
Figure 1. Incidence of Cardiovascular Disease by Coronary Artery Calcium (CAC) and Sex.2
In a separate study of adults ages 40 to 70 years with the incidental identification of coronary artery calcium identified on CT imaging, the amount of coronary artery calcium was again strongly associated with the future likelihood of a future cardiovascular event, as well as all-cause mortality (Table 4).3
Table 4. Risk of Cardiovascular Event and All-Cause Mortality by Coronary Artery Calcium (CAC)3
| CAC = 0 | CAC 1 to 100 | CAC 101 to 400 | CAC 400+ | |
| Cardiovascular Event, Hazard Ratio | 1.0, Reference | 8.3 | 31.1 | 52.0 |
| All-Cause Mortality, Hazard Ratio | 1.0, Reference | 0.7 | 1.5 | 1.6 |
The Benefits of Reversing Atherosclerosis
To assess the relationship of atherosclerotic plaque regression, or the partial reversal of atherosclerosis, researchers performed a systematic review and meta-regression analysis involving more than 6,000 patients in 17 prospective studies. Using intravascular ultrasound (IVUS) to assess the effect of atherosclerotic plaque volume and cardiovascular disease, it was demonstrated that that each 1% reduction in atherosclerotic plaque volume was associated with an 18% reduction in major adverse cardiovascular events, including heart attack, stroke, and death from cardiovascular disease (Odds Ratio: 0.82, 95% Confidence Interval: 0.70, 0.95, p = 0.011).1 Among the 17 studies included in this meta-regression analysis, some studies reported an average atherosclerotic plaque volume reduction as much as 5.0%, while the majority of positive trials demonstrated reductions closer to 1.0% and 2.5%.1,4
While the total volume of atherosclerotic plaque reduction is numerically modest, the favorable clinical impact is dramatic. This is likely attributed to the fact that atherosclerotic plaque reduction is a manifestation of several favorable changes occurring simultaneously, including the improvement of risk factors that halt or significantly reduce the progression of new atherosclerosis, the stabilization of existing atherosclerotic plaque, and the visualized reduction in atherosclerotic plaque volume. Notably, a reduction in cardiovascular disease can be achieved without atherosclerotic plaque reduction, highlighting the positive impact of atherosclerotic plaque stabilization, irrespective of plaque volume.7
How To Reverse Atherosclerosis
Numerous studies have demonstrated that both lifestyle interventions and prescription medications can promote the reversal of atherosclerosis, often referred to as atherosclerotic plaque regression. These strategies include aerobic exercise, the targeted lowering of atherogenic lipoproteins including LDL-C and Apolipoprotein-B (ApoB), Icosapent Ethyl (Vascepa), Angiotensin Receptor Blockers (ARBs), Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitors, and Colchicine. Additionally, we will review the preliminary data regarding Glucagon-like Peptide 1 (GLP-1) Receptor Agonist therapy. Notably, some of these strategies have been rigorously tested in randomized clinical trials, while others have only been characterized in observational studies and warrant further scientific assessment.
Aerobic Exercise
Regular aerobic exercise has been demonstrated to achieve coronary plaque regression, largely through its beneficial effects on vascular health and lipoprotein metabolism. To assess the impact of high-intensity interval training (HIIT) on coronary artery plaque volume, researchers enrolled 60 patients with known coronary atherosclerosis into two groups, one that participated in two weekly sessions of supervised HIIT at 85-95% of peak heart rate (n = 30), and a control group recommended to follow contemporary preventive guidelines (n = 30).5 Using intravascular ultrasound, the coronary arteries of study participants were examined at 6-months to assess changes atherosclerotic plaque volume. At the completion of this 6-month randomized clinical trial, the HIIT group achieved a 1.2% reduction in atherosclerotic plaque volume compared to plaque stability in the control group (between-group difference -1.4%, 95% CI: -2.7 to -0.1, P = 0.036).5
To assess the comparative effect of moderate continuous aerobic exercise training (MCT) and aerobic interval training (AIT), a separate randomized clinical trial was performed in 36 participants with established cardiovascular disease. Participants were randomly assigned to either the MCT or AIT group, to be performed three times per week for 12 weeks.6 The MCT protocol was defined as continuous walking or light running for 46 minutes at 70% of maximum heart rate (HR). The aerobic interval training (AIT) protocol consisted of a 10 minute warm-up followed by intervals of 4-times and 4-minutes, with an active pause of 3-minute in-between intervals. AIT target HR was 85% to 95% of the peak HR during intervals. Calorie consumption was comparable between groups. At the completion of the 12-week study period, intravascular ultrasound was used to examine the coronary arteries, for which the beneficial changes in atherosclerotic plaque volume were comparable between the two modalities of aerobic exercise. Specifically, there was a median reduction in atherosclerotic plaque volume of 2.3% and similar reductions in the atherosclerotic necrotic core (AIT -3.2%, MCT -2.7%, p <0.05). In conclusion, both MCT and AIT achieved atherosclerotic plaque regression and improvement in atherosclerotic plaque morphology. Furthermore, coronary artery plaque volume and composition did not differ significantly between patients who underwent AIT or MCT, suggesting that both aerobic exercise modalities are effective strategies for achieving atherosclerotic plaque regression and stabilization.6
Mechanistically, it is believed that aerobic exercise stabilizes atherosclerotic plaque and reduces cardiovascular risk by reducing systemic inflammation (e.g., decreased IL-6, TNF-α, CRP), enhancing endothelial function through increased nitric oxide bioavailability, and promoting favorable plaque composition changes, such as thickening the fibrous cap and reducing the necrotic lipid core. Furthermore, it has also been demonstrated that aerobic exercise upregulates antioxidant enzyme activity (e.g., superoxide dismutase, glutathione peroxidase), reduces oxidative stress and circulating levels of oxidative lipoproteins, and simultaneously promotes vascular repair through an upregulation of circulating endothelial progenitor cells (EPCs). Collectively, these physiological responses to aerobic exercise contribute to plaque stabilization, reduced risk of cardiovascular disease, and modest improvements in atherosclerotic plaque volume.
LDL-C Lowering Strategies
With regard to atherosclerotic plaque regression, the most well studied medications involve the targeted lowering of LDL-C and ApoB-containing lipoproteins. This includes statin therapy, PCSK9-inhibitor therapy, and Ezetimibe. Meanwhile, there are additional classes of medications that reliably lower LDL-C/ApoB, such as Bempedoic Acid, however, they have not been specifically evaluated in their ability to achieve atherosclerotic plaque regression.
Statin Therapy
Numerous studies have demonstrated the ability of statin therapy to achieve partial reversal of atherosclerosis (Table 5).7,8,9 Notably, Moderate Intensity statin therapy appears to be less effective than High Intensity statin therapy at achieving atherosclerotic plaque regression, most clearly demonstrated in the REVERSE Trial.9 Using LDL-C as a therapeutic end-point, a 25% reduction in LDL-C to an average level of 110 mg/dL demonstrated progression of coronary atherosclerosis, whereas a 46% reduction to LDL-C levels below 80 mg/dL achieved a 2.7% plaque reduction.9 Among the studies reviewed, statin therapy that reduced LDL-C below 80 mg/dL consistently achieved some degree of atherosclerotic plaque regression, ranging from 1% to 2.7% (Table 5).
Table 5. Comparative Effects of Statin Therapy, Atheroma Reduction, and LDL-C Reduction7,8,9
| ASTEROID Trial | SATURN Trial | SATURN Trial | REVERSAL Trial | REVERSAL Trial | |
| Statin Therapy | Rosuvastatin 40mg | Rosuvastatin 40 mg | Atorvastatin 80 mg | Pravastatin 40 mg | Atorvastatin 80 mg |
| Atheroma Reduction | – 1.0% | -1.2% | -1.0% | Progression | -2.7% |
| LDL-C Reduction | 53.2% | 62.6% | 56.8% | 25% | 46% |
| LDL-C Achieved | 60.8 mg/dL | 62.6 mg/dL | 70.2 mg/dL | 110 mg/dL | 79 mg/dL |
PCSK9 Inhibitor Therapy
Similar to statin therapy, multiple studies have demonstrated the ability of PCSK9 inhibitors, including Evolocumab (Repatha) and Alirocumab (Praluent), to achieve coronary atherosclerotic plaque regression.10,11 Briefly, PCSK9 inhibitor therapy, or Proprotein Convertase Subtilisin/Kexin Type 9 inhibitors, are a class of drugs that reduce LDL cholesterol by increasing the number of receptors available to clear LDL from the bloodstream. These medicines are administered by subcutaneous injection into the skin once or twice monthly, and represent a newer generation of lipid lowering therapy with comparison to statin therapy. Notably, PCSK9 inhibitors are potent in their ability to achieve significant reductions in LDL-C/ApoB, making them particularly effective for patients who cannot achieve adequate LDL-C reduction with statins alone, those with familial hypercholesterolemia, and those with intolerance to statin or other lipid-lowering therapy.
Table 6. Comparative Effects of PCSK9 Inhibitor Therapy, Atheroma Reduction, and LDL-C Reduction10,11
| GLAGOV Trial | PACMAN-AMI Trial | |
| PCSK9 Inhibitor Therapy Added to Statin Therapy | Evolocumab (Repatha) | Alirocumab (Praluent) |
| Atheroma Reduction | -1.0% | -2.1% |
| LDL-C Reduction | 59.8% | 84.8% |
| LDL-C Achieved | 36.6 mg/dL | 23.6 mg/dL |
Ezetimibe
In addition to statin and PCSK9 inhibitor therapy, Ezetimibe is another commonly utilized prescription medication for the targeted lowering of LDL-C, ApoB, and cardiovascular disease. Briefly, Ezetimibe functions to inhibit a protein in the small intestine, and reduces dietary and biliary cholesterol absorption, which functions to lower circulating LDL-C levels.
A variety of trials have evaluated the comparative effectiveness of Ezetimibe with Statin therapy compared to Statin therapy alone. The results have been mixed, with multiple trials demonstrating no additional benefit in plaque regression with the addition of Ezetimibe to Statin therapy.26,27] Meanwhile, there is limited evidence demonstrating an increased likelihood of achieving atherosclerotic plaque regression with the addition of Ezetimibe.12,28,29
In the PRECISE-IVUS Trial, the effectiveness of Atorvastatin monotherapy was compared to Atorvastatin with Ezetimibe with regard to atherosclerotic plaque regression.12 A total of 202 patients with established cardiovascular disease were randomized to Atorvastatin alone or Atorvastatin plus Ezetimibe. The dose of Atorvastatin was titrated to achieve an LDL-C of 70 mg/dL, with half of the study participants then randomized to receive the addition of Ezetimibe 10 mg once daily. Using intravascular ultrasound, coronary atherosclerotic plaque was evaluated at 9 to 12 months. The combination of Atorvastatin and Ezetimibe resulted in lower LDL-C and greater reductions in coronary atherosclerotic plaque regression (Table 7).
Table 7. Effectiveness of Ezetimibe and Atheroma Regression12
| Atorvastatin | Atorvastatin + Ezetimibe | |
| Atheroma Reduction | -0.3% | -1.4% |
| LDL-C Achieved | 73.3 mg/dL | 63.2 mg/dL |
| Percent of Patients With Coronary Plaque Regression | 58% | 78% |
Vascepa (Icosapent Ethyl)
Vascepa (Icosapent Ethyl) has garnered an increased amount attention following the success of the REDUCE-IT trial, where a highly purified formulation of the omega-3 fatty acid, Eicosapentaenoic Acid (EPA), known commercially as Vascepa (Icosapent Ethyl), achieved a significant reduction in cardiovascular disease among those with elevated triglycerides.30
In the Cherry Trial, 193 patients with established cardiovascular disease were randomly assigned to receive Pitavastatin alone or Pitavastatin and Eicosapentaenoic Acid (EPA).14 Both intervention groups demonstrated a reduction in plaque volume, with a greater number of patients achieving plaque regression in the group receiving Pitavastin and EPA (Table 8). Notably, elevated triglycerides were not an inclusion requirement of study participation, for which it appears some individuals derived possible benefit from EPA in the absence of elevated triglycerides. Meanwhile, there are potential side-effects of high-dose EPA therapy, warranting careful consideration of the appropriateness of this medication/supplement, a conversation that should be guided by a licensed healthcare professional.
Table 8. Effectiveness of Eicosapentaenoic Acid (EPA) and Atheroma Reduction14
| Pitavastatin | Pitavastatin + EPA | |
| Atheroma Reduction | -2.6 mm3 | -12.3 mm3 |
| Percent of Patients With Coronary Plaque Regression | 61% | 81% |
In a separate study, the EVAPORATE Trial assessed the effect of Vascepa (Icosapent Ethyl) on coronary atherosclerosis in patients with established cardiovascular disease and elevated triglycerides (135-499 mg/dL) while taking statin therapy.13 After 18 months, Icosapent Ethyl plus Statin therapy achieved a greater reduction in total plaque volume as well as fibrous low-attenuation plaques, suggesting an additional plaque-stabilizing effect. Despite the encouraging results, some concerns have been raised regarding important limitations of the trial, including the relatively large increase in plaque volume among the placebo group receiving mineral oil treatment. While some concern was raised regarding the possibility of the mineral oil placebo contributing to inflammation and atherosclerotic plaque progression, a post-hoc analysis did not support this hypothesis.31 At this time, regarding cardiovascular disease, Icosapent Ethyl (Vascepa) is only FDA approved for those with pre-existing cardiovascular risk factors and elevated triglycerides. Further studies evaluating the potential ability of EPA to achieve atherosclerotic plaque regression are warranted.
Antihypertensive Therapies
Clinical trials have demonstrated the ability of several blood pressure lowering medications to reduce cardiovascular disease. This includes Angiotensin-Converting Enzyme (ACE) Inhibitors, Angiotensin II Receptor Blockers (ARBs), Calcium Channel Blockers, Beta-Blockers, and Thiazide diuretics. Interestingly, several trials have demonstrated improvements in cardiovascular disease independent of blood pressure reduction, highlighting the possibility that some of these medications may achieve plaque stabilization. While several medications have demonstrated the ability to achieve plaque stabilization, the ability to induce atherosclerotic plaque regression has been most clearly demonstrated in trials evaluating Angiotensin Receptor Blockers (ARBs).
In one trial involving 100 patients with high blood pressure (hypertension) and established cardiovascular disease, participants were randomized to receive Olmesartan or Valsartan in addition to other guideline directed treatment.15 At six months, intravascular ultrasound was used to assess coronary artery plaque volume, for which both blood pressure lowering agents demonstrated a 4.7% reduction in coronary atherosclerosis, with no statistically significant difference between the two groups. Potential mechanisms to explain the observed benefits of ARBs include their ability to reduce vascular inflammation and increase collagen content in atherosclerotic plaque, both of which likely contribute to plaque stabilization and potential regression.
Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitors
While Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitors were initially developed for the treatment of diabetes, there is emerging evidence to suggest that these medications may contribute to atherosclerotic plaque regression.16 Among patients with type 2 diabetes, a prospective cohort study of 236 patients evaluated longitudinal changes in coronary atherosclerosis using coronary computed tomography angiography (CCTA). After a median duration of 14.6 months, it was demonstrated that SGLT2 inhibitor therapy was associated with significant reductions in overall plaque volume.16 While these findings persisted after the adjustment of cardiovascular risk factors and other medications, it is necessary to acknowledge that this trial was a prospective cohort study, rather than a randomized clinical trial, highlighting the need for further scientific evaluation.
While we await additional trials regarding SGLT2 Inhibitor therapy and atherosclerotic plaque regression, there is existing evidence demonstrating the ability of SGLT2 Inhibitor therapy to improve plaque stabilization through its ability to reduce peri-atherosclerotic inflammation, increase fibrous cap thickness, and reduced lipoprotein accumulation.17,18
Colchicine
Colchicine, a prescription medication with anti-inflammatory properties, has demonstrated potential utility in its ability to achieve atherosclerotic plaque regression and stabilization in human subjects.
In a prospective observational study spanning 12-months, low-dose colchicine was evaluated in 80 patients with a recent diagnosis of cardiovascular disease involving the coronary arteries.19 Among patients that received colchicine, there was a greater reduction in plaque volume than those who received routine medical treatment without Colchicine. It was also noted that Colchicine led to a significant decrease in high-sensitivity C-reactive protein (hsCRP) by 37.3% compared to 14.6% in the control group (p < 0.001), with no significant differences in LDL-C reduction between groups.
While we await randomized clinical trials to test this observation, the COLOCT randomized clinical trial convincingly demonstrated that Colchicine improved atherosclerotic plaque stabilization, measured by an increased fibrous cap thickness, reduced the lipid accumulation, and reduced macrophage infiltration in coronary plaques, measure by optical coherence tomography.20 Separately, in the COCOMO-ACS study, longer-term Colchicine treatment (≥16 months) resulted in greater increases in fibrous cap thickness, suggesting that prolonged therapy may be necessary to achieve significant morphological changes.21
Glucagon-like Peptide 1 (GLP-1) Receptor Agonist
While Glucagon-like Peptide 1 (GLP-1) Receptor Agonist therapy has attracted tremendous excitement for its effectiveness in improving obesity and a wide variety of obesity-related health issues, there are no trials that have evaluated the impact of GLP-1 Agonist therapy and atherosclerotic plaque regression in human subjects. While we await scientific investigation to test this possibility, GLP-1 Receptor Agonists have been demonstrated to reduce atherosclerotic plaque size and improved plaque stability in insulin-resistant mice.22 Similarly, other researchers have found that GLP-1 Receptor Agonist treatment prevented plaque progression and promoted plaque stability in rabbits with dyslipidemia.23
Follow-up Assessment of Atherosclerosis Using Non-Invasive Cardiovascular Imaging
Surveillance of coronary atherosclerosis with coronary computed tomography angiography (CCTA) is not routinely recommended for general use, but it may be utilized in specific cases to monitor the progression of coronary artery disease or to assess response to therapies. Serial CCTA imaging is typically used in clinical trials or occasionally in high-risk patients. While improvements in imaging technology have resulted in lower radiation burden with CCTA imaging, serial CCTA will increase one’s cumulative radiation exposure may contribute to potential adverse health events. Limitations of CCTA include lower resolution than other imaging technologies, such as Intravascular ultrasound (IVUS), resulting in the potential for imaging artifacts resulting in the misclassification of atherosclerotic plaque composition. Intravascular ultrasound is another imaging technology used to assess coronary atherosclerosis, however, given it’s invasive nature, it is primarily used for the sake of cardiovascular research, rather than clinical practice.






Hello Dr. Forey,
I enjoyed this article, and I wanted to add these comments regarding the use of icosapent ethyl (VASCEPA®) as a strategy to reduce atherosclerotic plaque and thus CVE risk reduction.
In the article you made note that a 1% reduction in plaque volume is associated with an 18% reduction in major cardiovascular events. You identified the relatively large plaque reduction in the EVAPORATE Trial (Total plaque regression = 9%) vs. -2.13% regression for alirocumab in the PACMAN-AMI Trial.
I wanted to point out that the 1% plaque reduction to 18% CVE RR relationship seems to hold out in the results of the ODYSSEY Trial (15% CVE RRR) and REDUCE-IT PCI (34% RRR). https://www.ahajournals.org/doi/full/10.1161/JAHA.121.022937
Also, your cautionary note regarding potential SAE’s in the use of IPE seems a bit overstated. in particular the reported event rate for hospitalization for AFIB in the IPE arm (3.1%) vs. the placebo arm (2.1%) are very modest when compared to the robust results of the REDUCE-IT Trial (Primary Composite End Point, 25% RRR, NNT 21).
https://www.ahajournals.org/doi/10.1161/JAHA.121.026756#:~:text=The%20absolute%20risk%20for%20serious%20bleeding%20associated%20with%20IPE%20in
Thank you very much for your thoughtful comment. I am glad to hear your enjoyed the article. I agree with everything you said. If I can clarify myself regarding Vascepa and other variations of high-dose EPA, I was trying to acknowledge that the data is preliminary and further research would be helpful to clarify the potential benefits (or lack thereof) versus potential harms (albeit modest). The results from REDUCE-IT were compelling. However, further research regarding plaque regression and stabilization, including in those without elevated triglycerides, would be helpful and informative. Thank you again.