Lipoprotein(a): Overcoming the Risk of Elevated Lp(a)
Introduction
Lipoprotein(a), abbreviated as Lp(a), has emerged as a significant risk factor of cardiovascular disease. Specifically, elevated levels of Lp(a) contribute to an increased risk of heart attack, stroke, aneurysm, cardiac valve dysfunction, and arterial blood clots.1,2 There is additional evidence demonstrating an association with worsened pregnancy outcomes and risk of pre-eclampsia.3,4 Given the autosomal dominant inheritance pattern, individuals with one affected parent have a 50% chance of inheriting elevated Lp(a) levels.5 While, the prevalence of elevated Lp(a) in the United States is estimated to be 15% to 24%, remarkably, as few as 0.3% of adults have had their Lp(a) levels tested, and less than 4% with a history of cardiovascular disease.6,7,8
Given the variety of cardiovascular complications associated with Lp(a), it is important to minimize cardiovascular risk in those with elevated Lp(a). Meanwhile, there are several notable challenges and unique considerations regarding the clinical management of Lp(a)-associated cardiovascular risk. Specifically, there are currently no FDA-approved medications for the targeted lowering of Lp(a). While we await the possibility of future treatment options, there is evidence to suggest potential usefulness of existing medications to modulate Lp(a)-related cardiovascular risk in select individuals.
Meanwhile, there is compelling evidence to suggest that a healthy diet, regular physical fitness, and healthy lifestyle choices can mitigate a significant amount of risk associated with Lp(a), independent of Lp(a) values.9,10 Furthermore, it has been shown that the presence of insulin resistance can increase Lp(a) levels as well the atherogenicity of Lp(a), highlighting the importance of maintaining optimal metabolic health.11,12 While Lp(a) levels are primarily determined by genetics, dietary restriction of refined sugars and highly processed carbohydrates has been shown to reduce Lp(a) by an average of 15%, with some individuals experiencing a 19.6% reduction in Lp(a) through dietary modification.13 Coupled with existing cardiovascular screening technologies, including computed tomography (CT) and ultrasound imaging, there are multiple effective strategies to reduce cardiovascular risk in those with elevated Lp(a).
The purpose of this article is to provide an optimistic, yet evidence-based perspective for those seeking to reduce Lp(a)-associated cardiovascular risk. This article will discuss Lipoprotein(a) in the context of cardiovascular disease, examine dietary and lifestyle strategies that have been shown to reduce Lp(a)-associated cardiovascular risk, address medication considerations, and proactive cardiovascular screening strategies.
Related Podcast Episode
Disclaimer
This content is for general educational purposes only and does not represent medical advice or the practice of medicine. Furthermore, no patient relationship is formed. Please discuss with your physician before making any dietary, lifestyle, or pharmacotherapy changes. Additionally, I have no financial conflicts of interest or affiliations with any diagnostic testing or pharmaceutical companies mentioned.
Notify Me of New Content
Provide your email address to receive notifications of new blog posts and podcast episodes.
Content Summary
Understanding Lipoprotein(a)
Lipoprotein(a) is structurally similar to low-density lipoprotein (LDL). However, Lp(a) consists of a variant LDL-particle that is bound to a unique protein called apolipoprotein(a). The structure of apolipoprotein(a) is what most profoundly differentiates Lp(a) from LDL, and contributes to its increased risk of inflammation, atherosclerosis, and blood clot formation.
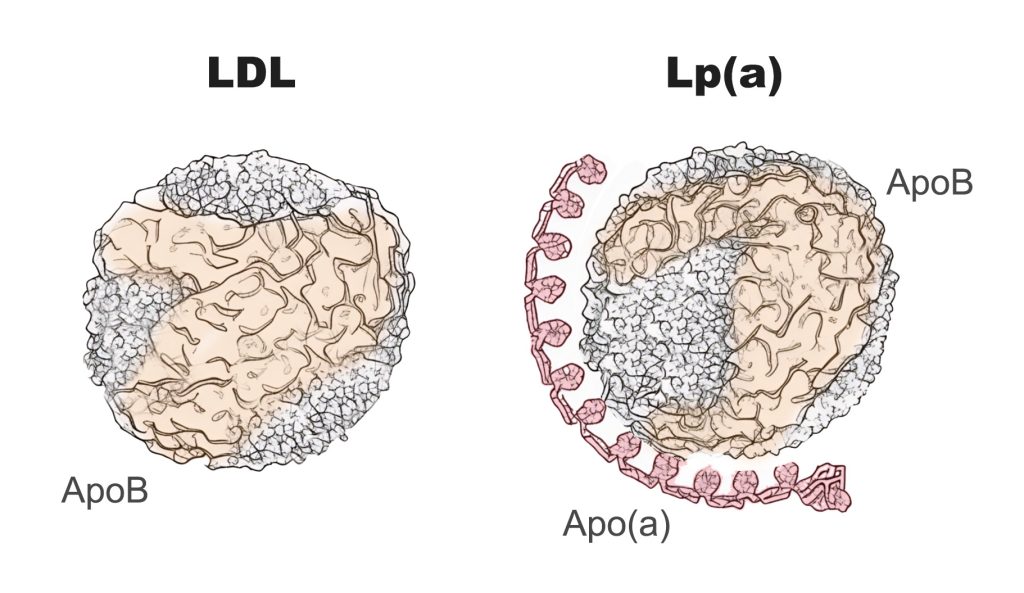
Figure 1. Low Density Lipoprotein (LDL) and Lipoprotein(a)
While Lp(a) and LDL both demonstrate the ability to penetrate the blood vessel wall and initiate the atherosclerotic process, apolipoprotein(a) exerts several unique mechanisms that augment Lp(a)’s negative impact on cardiovascular health. Specifically, it is estimated that each Lp(a) particle is 6.6x more atherogenic than LDL.14 One explanatory mechanism is that Lp(a) carries significantly more oxidized phospholipids than LDL, which contributes to a more pronounced inflammatory response within the blood vessel wall.2 Additionally, Lp(a) interferes with the body’s ability to dissolve clots (plasminogen mimicry), which increases the likelihood of blood clot and thrombus formation on atherosclerotic plaques.2 Lp(a) has also been shown to inhibit the production of nitric oxide, resulting in abnormal blood vessel tone and abnormal platelet aggregation.2
As previously mentioned, elevated levels of Lp(a) contribute to an increased risk of heart attack, stroke, aneurysm, cardiac valve dysfunction, and arterial blood clots.1,2 There is additional data suggesting a possible association with worse pregnancy outcomes and risk of pre-eclampsia.3,4 Multiple studies have demonstrated that as Lp(a) levels increase, the risk of these cardiovascular events also increases in a linear and continuous manner, without a plateau effect.12,15 In one study, individuals with Lp(a) levels greater than 75 nmol/L experienced a 5-fold higher risk of cardiovascular events than individuals with low levels of Lp(a), and individuals with Lp(a) levels greater than 125 nmol/L experienced a 10-fold higher risk.30 While Lp(a) is a notable cardiovascular risk factor, it is important (and refreshing) to acknowledge that the traditional cardiovascular and metabolic risk factors appear to be more strongly associated with the risk of cardiovascular disease (Table 1).31 Although it is recognized that each Lp(a) particle confers greater risk of cardiovascular disease than each individual LDL particle, the cumulative exposure of LDL is much greater than that of Lp(a). Meanwhile, risk factors of Metabolic Syndrome, including insulin resistance and high blood pressure, appear to be the most strongly associated risk factors of cardiovascular disease at all ages. Notably, the data presented in Table 1 is derived from the Women’s Health Study, a prospective cohort study examining cardiovascular outcomes in more than 28,000 women, but not men.
Table 1. Risk Factors of Cardiovascular Disease in the Women’s Health Study31
| Risk Factor, per SD Increment | Age of Onset < 55 Years Adjusted Hazard Ratio | Age of Onset 65 – 75 Years Adjusted Hazard Ratio |
|---|---|---|
| Insulin Resistance, LPIR Score | 6.40 | 2.09 |
| Systolic Blood Pressure | 2.24 | 1.48 |
| Triglycerides | 2.14 | 1.61 |
| Apolipoprotein B (ApoB) | 1.89 | 1.52 |
| C-Reactive Protein (CRP) | 1.76 | 1.62 |
| Non-HDL Cholesterol | 1.67 | 1.41 |
| Body Mass Index (BMI) | 1.47 | 1.33 |
| LDL Cholesterol | 1.38 | 1.24 |
| Hemoglobin A1c | 1.38 | 1.24 |
| Lipoprotein(a) | 1.22 | 1.11 |
Lipoprotein(a) Disproportionately Affects Young Individuals
While Lp(a) contributes to atherosclerosis in both young and old patients, its impact may differ based on age. Specifically, Lp(a) appears to have a larger potential impact on younger patients. For example, in one study of nearly 1,500 individuals diagnosed with cardiovascular disease, Lp(a) greater than 50 mg/dL was a profound risk factor of premature cardiovascular disease in young adults.16 However, this association was much less significant for individuals ages 45 to 60 years, and there was no statistically significant association among those older than 60 years.16
Table 2. Association of Elevated Lp(a) and Cardiovascular Disease By Age16
| ASCVD < 45 Years Odds Ratio ASCVD | ASCVD 45 – 60 Years Odds Ratio ASCVD | ASCVD > 60 Years Odds Ratio ASCVD | |
| Lp(a) >50 mg/dL | OR = 2.88 (1.7 – 4.6) | OR = 2.06 (1.4 – 3.2) | Not Statistically Significant |
In a second study that evaluated the prevalence of cardiovascular risk factors in those diagnosed with cardiovascular disease, the presence of elevated Lp(a) was more common among those who experienced premature cardiovascular disease before the age of 60 than those who were diagnosed with cardiovascular disease after the age of 60.32 Notably, the combination of elevated Lp(a) and Familial Hypercholesterolemia (FH) accounted for a minority of cardiovascular disease, although a larger proportion of premature cardiovascular disease.
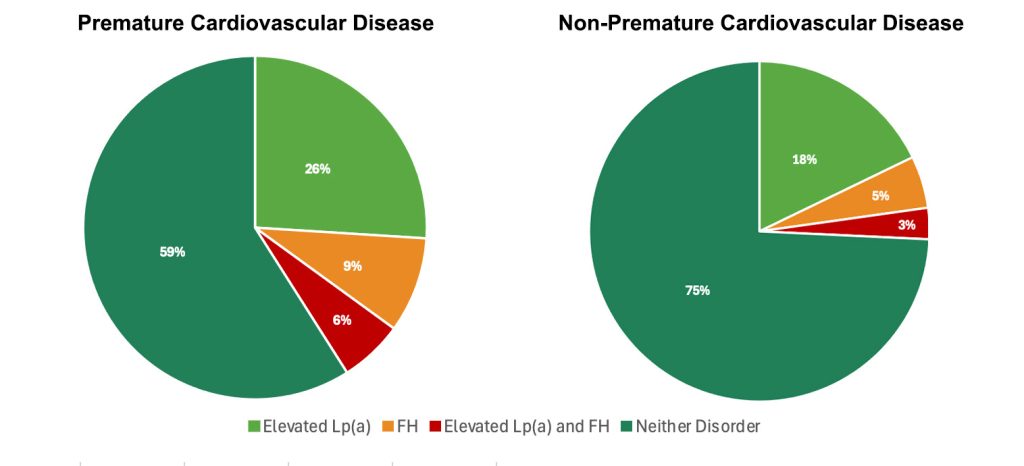
Figure 2. Frequency of elevated Lp(a) and FH in premature and non-premature cardiovascular disease.32
Premature cardiovascular disease defined as ASCVD before the age of 60 years. Abbreviations: FH, familial hypercholesterolemia; Lp(a), Lipoprotein(a).
Measuring Lipoprotein(a)
For those seeking to promote longevity and optimize cardiovascular risk factors, it is reasonable to obtain a Lipoprotein(a) measurement at least once. The traditional lipid panel typically includes measurements of total cholesterol, LDL-C, HDL-C, and triglycerides. Lp(a) is a distinct lipoprotein particle that is not routinely measured on the lipid panel, and is instead ordered as an additional test. The preferred unit of measurement for Lipoprotein(a) concentration is nmol/L because it standardizes the different particle sizes, allowing for more precise risk stratification than mg/dL.33 Meanwhile, many research studies report Lp(a) concentration in units of mg/dL. Therefore, this article will reference Lp(a) in both units of nmol/L and mg/dL.
Due to the similarities in the density of LDL and Lp(a), the cholesterol content of Lp(a) can contribute to the measured LDL-C. As a result, in individuals with elevated Lp(a), this can lead to an overestimation of LDL-C.34 Any individual with an elevated LDL-C should also have their Lp(a) level tested.
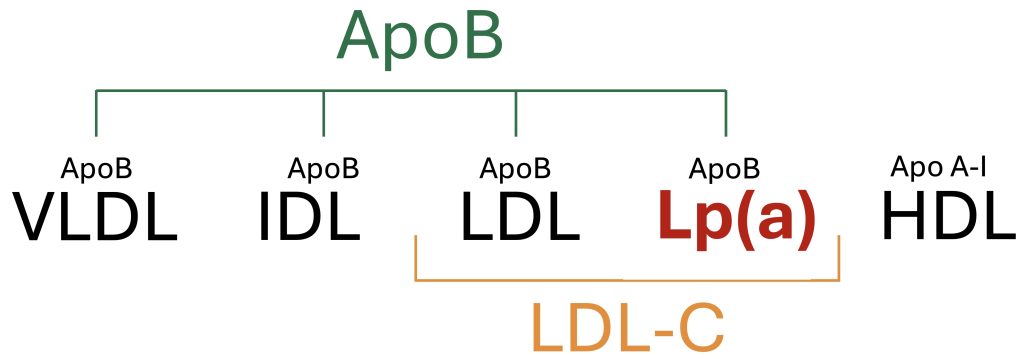
Figure 3. Overview of circulating plasma lipoproteins.
Elevated Lp(a): Definitions, Prevalence, and Heritability
Elevated Lp(a) levels are typically defined as those greater than 75 nmol/L (30 mg/dL), and levels greater than 125 nmol/L (50 mg/dL) are regarded as high risk.30 It is estimated that 20-25% of the global population has Lp(a) levels greater than 125 nmol/L.35
Lp(a) levels are predominantly influenced by genetics, with the heritability of Lp(a) estimated to account for 70% to 90% of Lp(a) concentrations.36 The LPA gene, which encodes apolipoprotein(a), plays a crucial role in determining Lp(a) levels. This strong genetic influence means that Lp(a) levels remain relatively stable throughout an individual’s life. However, it is recognized that several medical conditions may contribute to modest increases in Lp(a) levels throughout an individual’s lifetime.11 Meanwhile, there is some evidence that dietary intake can impact Lp(a) levels.13
Table 3. Risk Stratification of Lp(a) Levels
| Low Risk | Intermediate Risk | High Risk |
| Lp(a) < 75 nmol/L (<30 mg/dL) | Lp(a) 75 -125 nmol/L (30-50 mg/dL) | Lp(a) ≥125 nmol/L (≥50 mg/dL) |
Diabetes and Hypertension Are Associated With Increased Lp(a) Levels
While it is generally accepted that Lp(a) levels remain unchanged throughout an individual’s lifetime, there are medical conditions and risk-factors that appear to increase Lp(a) levels over time. Specifically, researchers evaluated Lp(a) levels in a cohort of more than 4,700 adults in the United States.11 Participants were categorized by baseline Lp(a) concentrations as Normal, Borderline-High (30-49 mg/dL), or High (≥50 mg/dL). Over the course of 15 years, 58.1% of participants with an initial Lp(a) level of Borderline-High (30-49 mg/dL) were detected to have a High Lp(a) level ≥50 mg/dL on follow-up testing. Among these adults with a ≥ 20 mg/dL Lp(a) level change between visits, a multivariate analysis demonstrated that diabetes, hypertension, and kidney dysfunction were three associated risk factors (Table 4).11 Participants with low Lp(a) or high Lp(a) at baseline tended to stay in these respective categories at 15-year follow-up testing.
Table 4. Risk factors associated with > 20 mg/dL increase in Lp(a) over 15-years.11
| Risk Factor | Odds Ratio |
| Diabetes | 1.39 (1.07-1.80, p=0.012) |
| Hypertension | 1.21 (CI 1.01-1.45, p=0.041) |
| Kidney Dysfunction (Albumin:Creatinine Ratio) | 1.11 (CI 1.03-1.19, p=0.004) |
Note: Hypertension compared to no hypertension; diabetes compared to no diabetes; kidney dysfunction defined as per natural log unit increment of albumin‐to‐creatinine ratio.
Diabetes Increases the Atherogenecity of Lp(a)
In a recent analysis of more than 27,000 participants in the United States, elevated levels of Lp(a) predicted cardiovascular disease similarly by sex, race, and ethnic background.12 However, in patients with diabetes, there was a nearly two-fold increase in the likelihood of experiencing cardiovascular disease compared to those without diabetes.12 Specifically, in individuals with an Lp(a) above 52.6 mg/dL (90th percentile), those with diabetes had a 51% higher likelihood of cardiovascular disease than those without diabetes, hazard ratio of 1.92 (95% CI: 1.50-2.45) and 1.41 (95% CI: 1.28-1.55), respectively. These findings demonstrate that diabetes likely exacerbates the atherogenic potential of elevated Lp(a) levels, making diabetic patients with high Lp(a) particularly susceptible to atherosclerosis and its complications. As previously discussed, diabetes also appears to be a risk factor that increases Lp(a) levels throughout an individual’s lifetime.11
Management of Elevated Lipoprotein(a) Levels
Managing elevated levels of Lp(a) is challenging due to its strong genetic basis and limited responsiveness to traditional interventions, including physical exercise and statin therapy. Importantly, however, there are several strategies that can be implemented to mitigate the associated cardiovascular risk of elevated Lp(a), independent of Lp(a) levels. In other words, meaningful reductions in cardiovascular disease can occur, even with stable Lp(a) levels. In this section, we will explore strategies to mitigate Lp(a)-associated cardiovascular disease and available therapies to lower Lp(a).
The Importance of Optimizing Healthy Lifestyle Habits
In a study of more than 14,000 individuals with elevated Lp(a), they were stratified into health categories of Poor, Intermediate, and Ideal based upon 7 health metrics. This included body mass index, diet quality, physical activity, tobacco use, blood pressure, hemoglobin A1c, and total cholesterol levels.17 Among these 7 health metrics, Poor health scores were assigned 0 points, Intermediate health scores were assigned 1 point, and Healthy scores were assigned 2 points. Based upon this scoring system of 7 health metrics, there was a maximum of 14 points corresponding to Healthy, and a minimum score of 0 points, corresponding with Unhealthy.
Individuals with elevated Lp(a) and a total of 0 points (Unhealthy) were used as the reference. In comparison to Unhealthy, the Intermediate category experienced a 35% reduction in cardiovascular disease, and the Healthy category experienced a 67% reduction.9 Most importantly, among individuals with an elevated Lp(a) in the Intermediate or Healthy categories, they demonstrated a lower likelihood of cardiovascular disease compared to those with low Lp(a) and Unhealthy behaviors (Figure 4).
Collectively, this data serves as reassurance for those seeking to minimize Lp(a)-associated cardiovascular risk in an era where there is limited ability to lower Lp(a). In other words, your genetics are not necessarily your destiny, and the health behaviors within your control can have a profound influence on reducing the likelihood of cardiovascular disease.
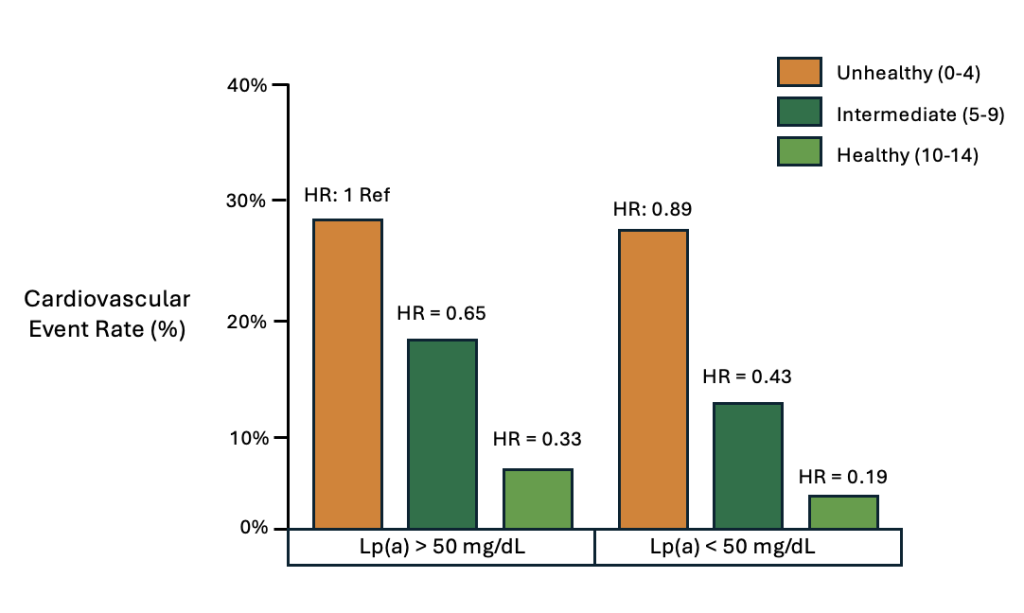
Figure 4. Cardiovascular disease event rates stratified by health categories and Lp(a).
In a separate study of more than 4,500 adults, the cohort was separated into two groups based upon baseline Lp(a) concentrations, and their lifestyle habits were evaluated on the basis of dietary quality, physical activity, smoking status, and body weight.10 A scoring system allocated 1 point for an ideal diet, 1 point for regular physical activity, 1 point for no tobacco use, and 1 point for a normal BMI. Similarly to the previous study, healthy lifestyle behaviors were strongly associated with a lower risk of cardiovascular disease, regardless of Lp(a) levels (Figure 5).
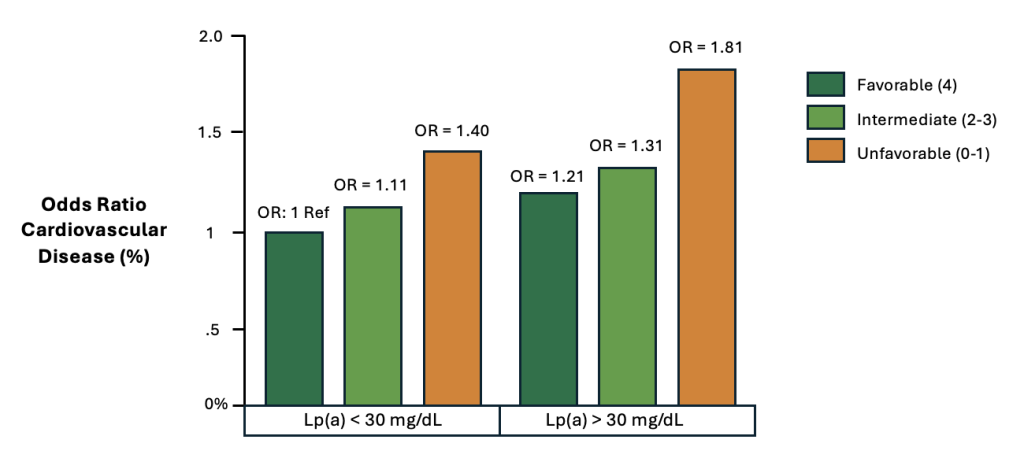
Figure 5. Cardiovascular disease stratified by lifestyle habits and Lp(a)10
The Importance of Maintaining Optimal Metabolic Health
As previously discussed, insulin resistance appears to (1) increase the atherogenicity of Lp(a) and (2) likely contributes to increasing Lp(a) concentrations throughout an adult’s lifetime.11,12 Additionally, insulin resistance measured by the LPIR Score appears to be the strongest risk factor of premature cardiovascular disease,31 highlighting the importance of avoiding insulin resistance and maintaining optimal metabolic health. While there are many successful approaches to a healthy dietary pattern, targeted restriction of refined sugars and highly processed carbohydrates is one effective strategy for the prevention and reversal of insulin resistance, even in the absence of caloric reduction.37
Regarding the effect of dietary intake and Lp(a) concentrations, a randomized clinical trial of 164 adults was conducted to assess the impact of three diets varying in carbohydrate intake. When carbohydrate restriction was limited to 20% of daily energy intake, Lipoprotein(a) was reduced by an average of 14.7%, with some individuals achieving as much as a 19.6% reduction in Lp(a).11 Additionally, the Low Carbohydrate group was the only diet tested that improved insulin resistance measured by the LPIR Score. Simply stated, improvements seen in insulin resistance corresponded with improvements observed in Lp(a). Mechanistically, this is consistent with the previous observation that diabetes is a risk factor for increased Lp(a) levels throughout one’s lifetime.11 Importantly, LDL-C values were similar in all diets tested. Furthermore, all diets achieved meaningful reductions in system inflammation measured by hs-CRP and IL-6.13
Importantly, the restriction of added and refined sugars and highly processed carbohydrates is not an endorsement of a low carbohydrate or ketogenic diet. Rather, the restriction of added and refined sugars and highly refined carbohydrates is a fundamental recommendation of all mainstream diets, including the Mediterranean diet, plant-based diet, whole-foods diet, and low carbohydrate diet. More broadly speaking, rather than the endorsement of any specific diet, it is an effective strategy to deliberately avoid the consumption of highly processed foods, for which 89.7% of added and refined sugar intake in the United States is derived from highly processed foods, as well as 75% of sodium intake.38,39 Meanwhile, it is important for health conscious individuals to work with their physician to objectively monitor their individual response to any dietary change(s). This includes the regular monitoring of trends in ApoB and the components of Metabolic Syndrome including body weight, waist circumference, insulin resistance, blood pressure, triglycerides, VLDL, and systemic inflammation. While HDL-C is not causally associated with cardiovascular disease, it is a fundamentally important marker of metabolic health, and is strongly associated with both cardiovascular and non-cardiovascular illnesses, including cancer, dementia, and infertility.
Table 5. Dietary Impact of Carbohydrate Restriction on Lipoprotein(a), LPIR Score, and LDL-P.13
| Low Carbohydrate (20% Carb, 21% Sat Fat) | Moderate Carbohydrate (40% Carb, 14% Sat Fat) | High Carbohydrate (60% Carb, 7% Sat Fat) | |
| Lipoprotein(a) | – 14.7% | – 2.1% | + 0.2% |
| Insulin Resistance, LPIR Score | – 5.3 | No Change | + 3.6 |
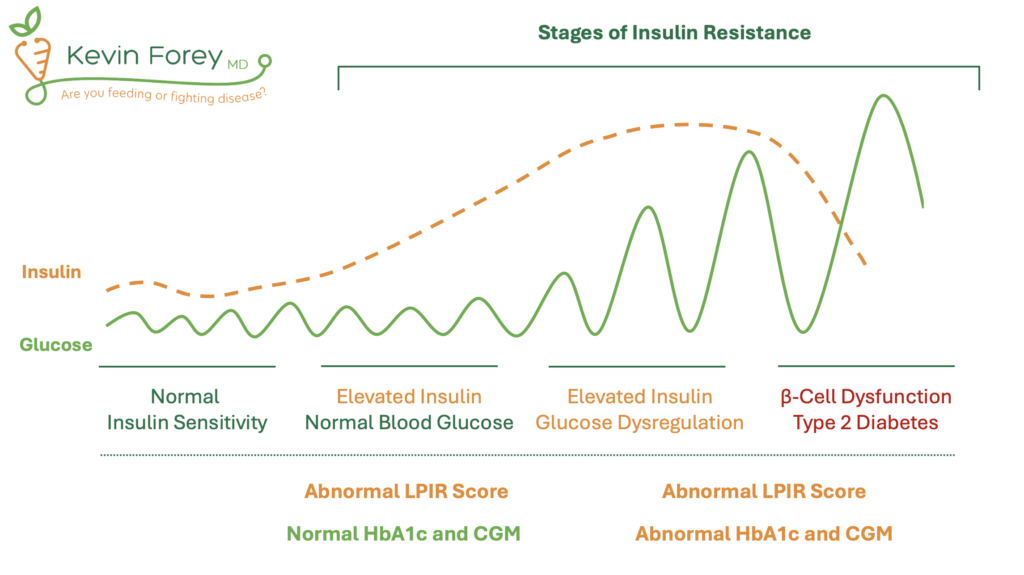
Figure 6. Stages of Insulin Resistance and the Relationship to Diagnostic Testing.
Aggressive LDL-C Lowering
In addition to optimizing healthy lifestyle habits, aggressive lowering of LDL-C and Apolipoprotein-B (ApoB) are effective strategies for further reducing cardiovascular risk among individuals with elevated LDL-C. Given the limited options for directly lowering Lp(a), the reduction of LDL-C and ApoB remain a primary strategy for those with elevated concentrations of Lp(a). The following section will focus on the use of prescription medications, however, the reduction of saturated fat and increased consumption of dietary fiber have both been demonstrated to lower LDL-C without the use of prescription medications.
Statin Therapy
In a trial of 146 men with elevated LDL-C at baseline, participants were randomized to a variety of trials intended to lower LDL-C and ApoB, including Lovastatin 40 mg once daily.18 Trial participants were then assessed based upon their percent of LDL-C reduction and baseline Lp(a) concentrations. Among participants with an LDL-C reduction greater than 10%, dramatic reductions in cardiovascular disease were observed in those with elevated Lp(a) values at baseline. (Figure 7) Specifically, among the patients with elevated Lp(a) and an LDL-C reduction greater than 10%, there was a 77% reduction in the likelihood of cardiovascular disease compared to those with elevated Lp(a) but a minimal response to LDL-C lowering therapy (RR = 0.23; 95% CI 0.06 to 0.99).18 Impressively, the achieved LDL-C was 120 mg/dL in the group with the “substantial LDL-C reduction,” demonstrating two important points: (1) modest to moderate reductions in LDL-C appear to achieve significant reductions in cardiovascular disease among those with elevated Lp(a), and (2) presumably the risk of cardiovascular disease can be further reduced by achieving LDL-C values below 120 mg/dL.
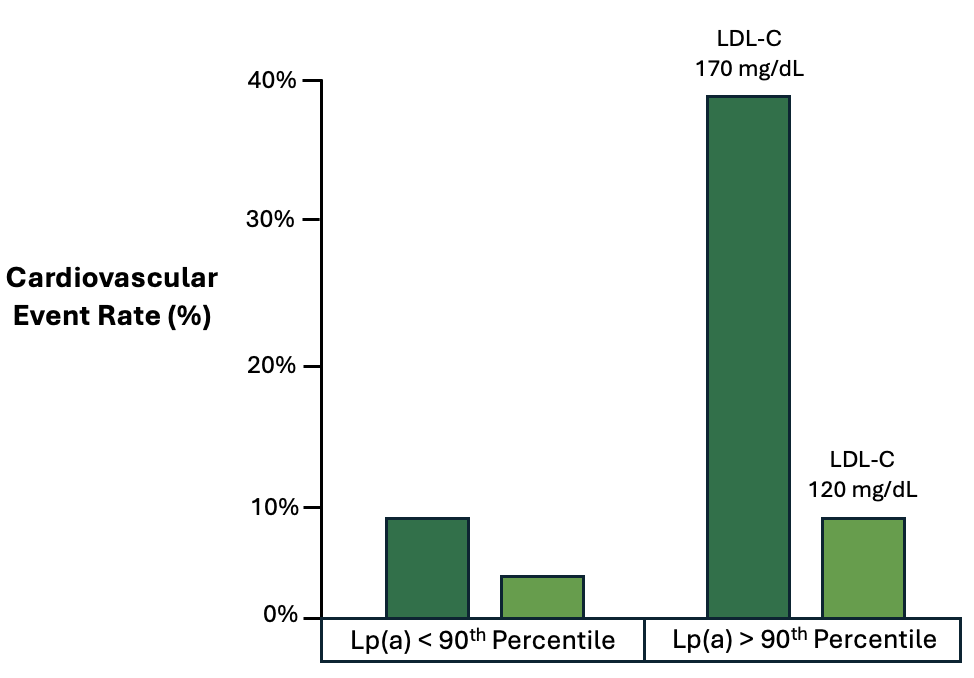
Figure 7. Relationship of LDL-C treatment response and cardiovascular event rates.18
Lp(a) 90th percentile was 46.2 mg/dL or approximately 99 nmol/L.51
Ezetimibe
Ezetimibe does not affect Lp(a) levels appreciably. Specifically, in one meta-analysis of 10 trials and more than 5,000 participants, there was no effect on Lp(a) concentrations with Ezetimibe monotherapy or in combination with statin therapy.22 Meanwhile, Ezetimibe is an effective strategy for lowering LDL-C and cardiovascular risk. At this time, I am not aware of any trials that have evaluated cardiovascular risk reduction with Ezetimibe in patients with elevated Lp(a).
Bempedoic Acid
Similarly, Bempedoic acid does not significantly lower Lp(a) levels. In multiple trials, Bempedoic acid has minimal effect on Lp(a) levels, despite its reduction in LDL-C, hs-CRP, and other inflammatory markers. This finding is consistent across multiple studies and meta-analyses. Therefore, while Bempedoic acid is effective for lowering LDL-C and reducing cardiovascular risk, it does not impact Lp(a) levels directly.23,24,25
Therapies Capable of Lowering Lp(a) and LDL-C
PCSK9 Inhibitors
PCSK9 Inhibitor therapy is a newer and more sophisticated treatment used for the reduction of elevated LDL-C in patients with a high risk of cardiovascular disease and/or intolerance to other more commonly utilized lipid lowering therapies. Evolocumab (Repatha) and Alirocumab (Praluent) are the two most commonly used PCSK9 inhibitor therapies in clinical practice. They are administered as subcutaneous injections into the skin once or twice per month, depending on the prescribed dose and frequency.
While PCSK9 inhibitor therapies are capable of achieving dramatic reductions in LDL-C by as much as 45-60%, they have also demonstrated the ability to lower Lp(a) by 15-30%.19,20 Upon review of the ODYSSEY trials testing Alirocumab (Praluent), each 25% reduction in Lp(a) resulted in a 12% relative risk reduction in major adverse cardiovascular outcomes.21 However, this association was not statistically significant after adjusting for changes in LDL-C levels, suggesting that the benefits might be primarily mediated through LDL-C reduction rather than Lp(a) reduction.21
Interestingly, however, in the analysis of the FOURIER trial involving Evolocumab (Repatha), it was demonstrated that higher baseline Lp(a) levels were associated with an increased risk of cardiovascular events, and that patients with higher baseline Lp(a) levels experienced greater absolute reductions in cardiovascular events.52
While we await further clinical trials clarifying the unique impact and effect of PCSK9 inhibitor therapy on Lp(a)-associated cardiovascular disease, it is apparent that PCSK9 inhibitor therapies are effective at reducing the risk of cardiovascular disease in individuals with elevated cardiovascular risk, including those with elevated Lp(a). At this time, PCSK9 inhibitor therapy is only FDA approved for lowering LDL-C in those with hypercholesterolemia, high risk of cardiovascular disease, and/or intolerance to other lipid lowering therapies. Evolocumab (Repatha) and Alirocumab (Praluent) are not approved for the targeted lowering of Lp(a).
At this time, the primary barriers to more widespread usage of PCSK9 inhibitor therapy include limited indications for and insurance coverage of these medications, as well as the cost associated with these therapies.
Niacin (Nicotinic Acid)
Niacin, also known as vitamin B3, is a vitamin that can favorably influence LDL-C, triglycerides, and HDL-C. Notably, it has also been demonstrated to reduce Lp(a) by as much as 20-30%.53,54,55 Although Niacin can lower Lp(a) levels, its usage has diminished profoundly due to its limited impact on cardiovascular outcomes and the prevalence of side effects, including flushing, itching, gastrointestinal disturbances, and liver toxicity.56 While Niacin improves circulating lipoproteins, it has not been demonstrated to be a reliable treatment strategy for the reduction of cardiovascular events. Specifically, in the AIM-HIGH trial, Lp(a) levels were reduced, however, Niacin did not achieve reductions in cardiovascular events in patients with established cardiovascular disease.57 Similarly, the HPS2-THRIVE trial also failed to show a benefit of Niacin on cardiovascular outcomes when added to statin therapy.58 Therefore, at this time, no major cardiovascular guidelines recommend the routine usage of Niacin for the sake of lowering Lp(a) or cardiovascular disease in at-risk individuals.
Lipoprotein Apheresis
The only treatment currently approved for lowering Lp(a) is lipoprotein apheresis. Apheresis is a process similar to dialysis, and involves drawing blood from the patient, passing it through a machine that separates and removes Lp(a) particles, and then returns the blood to the patient. This process is typically performed every 1-2 weeks, depending on the severity of Lp(a) elevation and the patient’s individual healthcare circumstances. This treatment is largely reserved for patients with genetic abnormalities, such as Familial Hypercholesterolemia (FH), and is not routinely prescribed outside of highly specialized clinical lipidology practices.
Aspirin For Those Without Cardiovascular Disease (Primary Prevention)
Aspirin is an antiplatelet agent that may serve a beneficial role among individuals with elevated levels of Lp(a). As previously discussed, Lp(a) interferes with the body’s ability to dissolve clots through a mechanism described as plasminogen mimicry. Aspirin functions to inhibit platelet aggregation, which may help to mitigate risk of cardiovascular disease in certain circumstances. Meanwhile, Aspirin’s antiplatelet effect also increases the risk of adverse bleeding, highlighting the delicate decision at hand regarding the relative risks and benefits of Aspirin in individuals without pre-existing cardiovascular disease (primary prevention).
While there are no randomized clinical trials testing Aspirin and its ability to reduce cardiovascular disease among individuals with elevated Lp(a), we will instead review the relevant data from two large prospective cohort studies, as well as a large retrospective analysis of National Health and Nutrition Examination Survey (NHANES) data.
In the Multi-Ethnic Study of Atherosclerosis (MESA), Aspirin use was associated with a significant reduction in cardiovascular disease among individuals with elevated Lp(a) levels (HR 0.54, CI 0.32-0.94).26 Similarly, secondary analysis of the ASPREE trial demonstrated that older individuals with elevated Lp(a)-associated genotypes experienced lower rates of cardiovascular disease with Aspirin use.27 Importantly, there were no meaningful increase in bleeding risk observed.27 Separately, in an analysis of nearly 3,000 individuals from the NHANES database, regular Aspirin use was associated with a 52% lower risk of cardiovascular mortality among individuals with elevated Lp(a) (HR=0.48, CI 0.28-0.83), but not for those without elevated Lp(a) (HR=1.01, CI 0.81-1.25).28
At this time, outcomes data from randomized trials is needed for further evaluation of Aspirin’s potential utility in reducing the risk of cardiovascular disease in individuals with elevated levels of Lp(a). Meanwhile, among individuals with high-risk of cardiovascular disease, Aspirin may assist in the primary prevention by reducing cardiovascular events, but this potential benefit must be weighed against the risk of bleeding. Any decision to initiate or discontinue Aspirin therapy should be guided by a licensed healthcare professional and monitored closely for adverse events.
Aspirin For Those With Cardiovascular Disease (Secondary Prevention)
Among individuals with a previous heart attack, stroke, or other forms of atherosclerotic cardiovascular disease, Aspirin has been demonstrated to reduce the risk of subsequent heart attack, stroke, and death (secondary prevention).59,60. Therefore, both the American Heart Association (AHA) and the American College of Cardiology (ACC) recommend Aspirin therapy for secondary prevention of cardiovascular disease in patients with a suitable bleeding-risk profile.61
With the emergence of Coronary Artery Calcium scoring (CAC), atherosclerotic cardiovascular disease can now be easily identified with non-invasive techniques before the onset of heart attack, stroke, or other complications of atherosclerosis. In a separate analysis of MESA data, it was demonstrated that individuals with CAC ≥100 had favorable risk/benefit outcomes for Aspirin use, with a lower number needed to treat (reduction of cardiovascular disease) compared to the number needed to harm (risk of bleeding).62,63 Therefore, in high-risk individuals with a CAC > 100, it may be reasonable to initiate Aspirin therapy from the standpoint of secondary prevention of pre-existing coronary artery disease. Again, however, any potential benefit of Aspirin therapy must be compared to the individual’s potential risk of bleeding, a conversation that should be guided by a licensed healthcare professional.
Emerging Therapies for Lowering Lipoprotein(a)
Emerging therapies to lower Lp(a) include antisense oligonucleotides (ASOs) and small interfering RNAs (siRNAs), both of which target the genetic production of apolipoprotein(a) to reduce Lp(a) levels. Pelacarsen (ASO), currently in Phase III trials, has shown up to an 80% reduction in Lp(a), while Olpasiran (siRNA) has demonstrated reductions up to 90%, both offering promising approaches for reducing cardiovascular risk.64
Gene-editing technologies, such as CRISPR/Cas9, represent a potential future avenue for Lp(a) reduction. Although still in early research stages, these therapies aim to directly modify the genes responsible for Lp(a) production, offering the possibility of a more permanent reduction in Lp(a) levels. However, clinical trials for this approach are not yet underway.
Finally, while PCSK9 inhibitors are currently being used to lower LDL-C, research is ongoing to explore their long-term impact on cardiovascular events in individuals with elevated Lp(a).
Non-Invasive Cardiovascular Disease Screening Strategies
Non-invasive cardiovascular screening techniques are valuable tools for assessing cardiovascular risk, particularly in asymptomatic individuals. The information gathered from these tests can help in the decision-making process of several important considerations, including the use of prescription medications. The following section will provide a brief overview of several non-invasive cardiovascular screening techniques, as well as a discussion regarding repeat screening intervals. Cardiac stress testing, echocardiography, and ankle-brachial index (ABI) are outside the scope of this article and will not be addressed.
Coronary Artery Calcium (CAC) Scoring
Coronary Artery Calcium (CAC) Scoring utilizes low-dose CT imaging to measure the amount of calcium in the arteries supplying blood to the heart (coronary arteries), which correlates strongly with the presence of atherosclerosis and risk of future heart attack. The primary purpose of CAC scoring is to assess the +/- presence of coronary atherosclerosis and risk of cardiovascular disease. Strengths of this imaging technique include the non-invasive nature, ability to quantify the amount of calcified plaque present, it is a strong predictor of future cardiovascular events, it is quick, easy, and can be affordable at select diagnostic imaging centers. Limitations of CAC scoring are the inability to detect non-calcified plaque (soft plaque) and the limited exposure of radiation to the patient.
While CAC scoring is an excellent tool for predicting the likelihood of future heart attack, the story is more complex in those with elevated Lp(a). Analysis of MESA data demonstrated that elevated Lp(a) is independently associated with cardiovascular events, even after adjusting for the CAC score.29 As a result, a CAC score of 0 does not eliminate the risk associated with elevated Lp(a), particularly in younger adults, in whom a CAC score of 0 may underestimate the lifetime risk of cardiovascular disease and provide false reassurance.
The 2019 ACC and AHA guidelines recommend CAC scoring for adults ages 40-75 years who are at intermediate risk for atherosclerotic cardiovascular disease.61 For individuals with an elevated cardiovascular risk, including those with an elevated Lp(a), early cardiovascular screening is reasonable to consider, perhaps closer to the age of 40. In a large study comprising more than 22,000 individuals, researchers sought to identify the optimal age for a first CAC scan in low-risk individuals. Interestingly, calcified atherosclerosis was present in 34% of study participants, with an average age 43.5 years.65
In the event that calcified atherosclerosis is detected, it would represent a clear indication for lipid lowering therapy. If no atherosclerosis/calcification is present, a decision to repeat CT imaging at 3-7 year intervals could be considered, guided by that individual’s risk factors. More specifically, it has been proposed that repeat CT can be considered at 5-7 year intervals for low-risk individuals, 3-5 years for intermediate risk individuals, and 3-years for high-risk individuals.66
Coronary CT Angiography (CCTA)
Coronary CT Angiography (CCTA) is a similar but more advanced CT scan than CAC scoring, which utilizing intravenous contrast through an intravenous (IV) access site, generally in the arm, which allows the radiologist to characterize more detailed images of coronary arteries, including the presence of both calcified and non-calcified plaques. Strengths of this test include the ability to detect soft plaque in addition to calcified plaque, and the degree of narrowing of cardiac blood vessels. Limitations of CCTA include a higher exposure of radiation to the patient, and the utilization of contrast material, which may not be suitable for patients with kidney dysfunction, and can also provoke an allergic/anaphylactic reaction. The timing to initiate screening and frequency of follow-up screening is similar for CCTA as CAC (discussed above).
Carotid Ultrasound
Carotid Ultrasound utilizes high-frequency sound waves to visualize the carotid arteries in the neck, which are the major arteries supplying blood to the brain. The purpose of this ultrasound test is to detect atherosclerotic plaque or narrowing (stenosis) of these arteries. The test can provide important information regarding the risk of stroke and it correlates with the risk of heart attack even though it does not visualize the coronary arteries of the heart.67 Strengths of carotid ultrasound include its non-invasive nature, the ability to detect soft and calcified plaque, no radiation exposure, and its correlation with atherosclerosis in the coronary arteries of the heart.67 Carotid Intimal Media Thickness (CIMT) is a specialized type of ultrasound test utilized by some. Due to its lower correlation with coronary artery disease and high degree of user variability, it is much less commonly utilized.
In an attempt to compare the usefulness and utility of carotid ultrasound to CAC, we will review data derived from the Progression of Early Subclinical Atherosclerosis (PESA) cohort study, which investigated the development and progression of atherosclerosis in healthy individuals.68 The PESA study used a variety of these non-invasive cardiovascular screening techniques, including CAC scoring and ultrasound imaging of multiple locations throughout the body. Notably, there was a dramatically higher likelihood of detecting atherosclerosis in the aorta, iliofemoral, and carotid arteries using ultrasound, with much lower rates of detection used CAC scoring (Figure 8). In other words, atherosclerosis outside of the coronary arteries was observed more frequently and at lower levels of LDL-C than coronary artery calcification. Specifically, in the majority of LDL-C ranges, atherosclerosis was detected in the carotid artery 2-3x more frequently than calcified plaque of the coronary arteries.
Notably, the AHA and ACC guidelines do not recommend carotid artery ultrasound for routine screening purposes of atherosclerosis in the same way as CAC scoring.61 In contrast, the European Society of Cardiology guidelines suggest that carotid ultrasound can be considered for risk stratification in intermediate-risk individuals.69 In summary, individuals with elevated Lp(a) would be intermediate- to high-risk individuals, and based upon data from PESA, carotid ultrasound identifies atherosclerosis more frequently and at lower LDL-C levels than CAC scoring. Therefore, an alternating pattern of screening with CAC scoring and carotid ultrasound can be considered in high-risk individuals. This seems particularly reasonable given the safe, non-invasive nature, and relative affordability of ultrasound imaging.
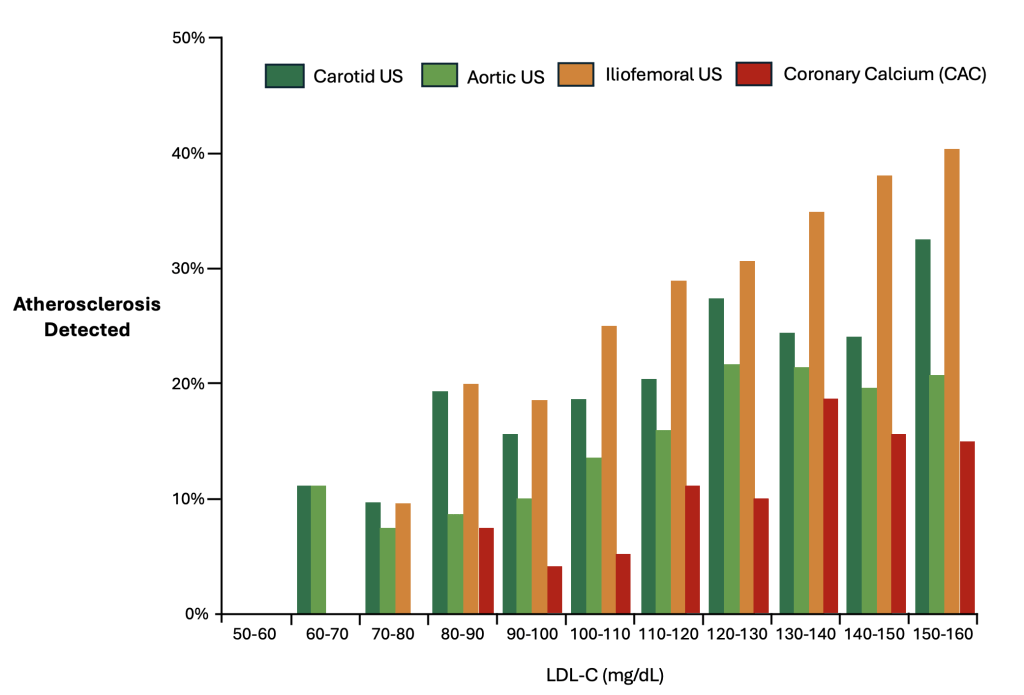
Figure 8. Relation Between LDL-Cholesterol Levels and Atherosclerosis by Vascular Territory68





Why do you not provide any background (that I can find) on who you are and your education?
Not great for evoking trust- just sayin’ 🙂
Rosemary MS, CNS, CN, LDN, IFMP
Hi Rosemary, thank you for your comment. I will include this relevant information on my website.
M.A. Biophysics – University of San Diego
M.D., M.B.A. – University of Colorado School of Medicine
Internal Medicine Residency – Mayo Clinic Arizona
Very thorough and helpful. Do you have any guidance on which level of intensity or preference of statin to use in the case of detecting lpa > 125 in a younger individual as a preventative means?
Thank you.