Beyond LDL-C: Why Remnant Cholesterol Deserves More Attention
Introduction
It is well established that low-density lipoprotein (LDL) contributes to the risk of cardiovascular disease and the development of atherosclerosis.1 However, with advancements in our understanding of cardiovascular disease, Apolipoprotein B (ApoB) has emerged as a stronger predictor of risk than LDL cholesterol (LDL-C).2-6 Unlike LDL-C, ApoB reflects the total number of atherogenic particles, making it a more comprehensive measure of cardiovascular risk. Aside from LDL, “remnant cholesterol” constitutes the majority of the remaining ApoB-containing particles. Notably, remnant cholesterol is an independent risk factor cardiovascular disease, even after accounting for LDL-C and ApoB levels.7 In fact, an emerging body of evidence suggests that remnant cholesterol may be an even stronger risk factor for cardiovascular disease than LDL-C.7-13 Despite its clinical relevance, many healthcare professionals and health-conscious individuals remain unfamiliar with remnant cholesterol and its causal role in atherosclerosis.
The distinction of LDL-C and remnant cholesterol is important because standard treatment strategies to lower LDL-C are often less effective at reducing remnant cholesterol. For example, unlike LDL-C, reductions in saturated fat intake do not meaningfully improve remnant cholesterol levels.14-17 Instead, remnant cholesterol is more strongly influenced by the intake of highly processed carbohydrates, refined grains, and foods with added sugars.14-18 Prescription drug therapies further reflect this difference, where statins primarily lower ApoB-containing particles through LDL receptor–mediated clearance, which is not particularly effective at removing triglyceride-rich remnants.19,20 As a result, statins are typically 2-3x more effective in their ability to lower LDL-C than remnant cholesterol.21-23
Importantly, remnant cholesterol remains a significant risk factor even among individuals with optimal LDL-C and ApoB levels, including those with LDL-C levels below 70 mg/dL.7,21,24 This finding challenges the common assumption that lowering LDL-C alone sufficiently addresses cardiovascular risk, and underscores the need for greater awareness of the residual risk attributed to remnant cholesterol. Therefore, the purpose of this article is to highlight remnant cholesterol as an often-overlooked risk factor of cardiovascular disease. In addition, evidence-based strategies to lower remnant cholesterol will be discussed. Finally, the article will explore the growing body of research linking elevated remnant cholesterol to several non-cardiovascular conditions, including Alzheimer’s disease,25,26 vascular dementia,25,27 type 2 diabetes,28,29 certain cancers,30-32 chronic inflammation,12 and autoimmune diseases.33-35
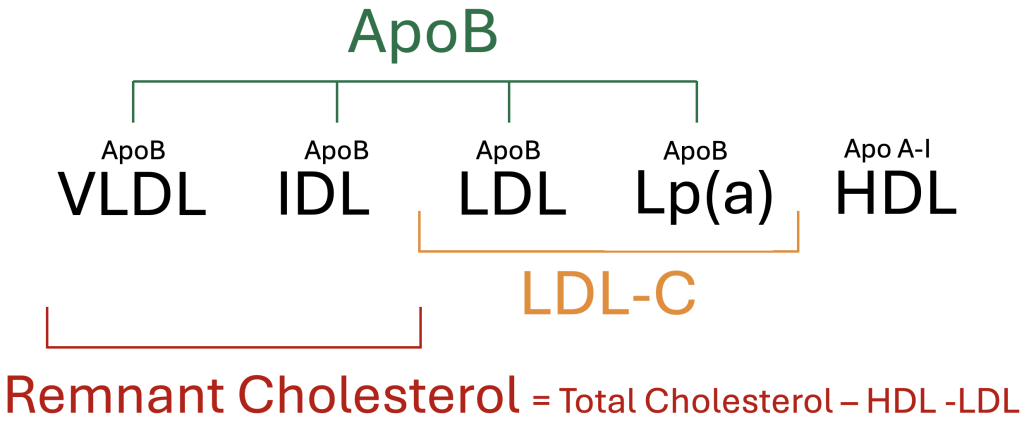
Remnant Cholesterol = Total Cholesterol – LDL – HDL
Related Podcast Episode
Disclaimer
This content is intended for general educational and informational purposes only and does not represent the practice of medicine, the giving of medical advice, diagnosis, or treatment. Furthermore, no patient relationship is established by accessing or interacting with this content. Individuals should consult their personal healthcare provider before making any changes to their diet, lifestyle, medications, or other aspects of their health. Additionally, I have no financial conflicts of interest or affiliations with any diagnostic laboratories, supplement manufacturers, or pharmaceutical companies referenced.
Notify Me of New Content
Provide your email address to receive notifications of new blog posts and podcast episodes.
Content Summary
What Is Remnant Cholesterol
Remnant cholesterol refers to the cholesterol contained within triglyceride-rich lipoproteins, specifically very low-density lipoproteins (VLDL), intermediate-density lipoproteins (IDL), and chylomicron remnants. These particles belong to the family of atherogenic lipoproteins that contain Apolipoprotein B (ApoB) but are distinct from low-density lipoproteins (LDL). While LDL-C reflects the cholesterol concentration within LDL particles alone, remnant cholesterol captures the cholesterol burden from triglyceride-rich particles. Although remnant cholesterol is not routinely reported on standard lipid panels, it can be easily estimated by subtracting LDL-C and HDL-C from the total cholesterol value.
Remnant Cholesterol = Total Cholesterol – LDL – HDL
Figure 1. Overview of circulating lipoproteins and common lipoprotein measurements
Remnant Cholesterol Calculator
Table 1. Remnant Cholesterol Thresholds
| Optimal | Elevated | High |
| < 20 mg/dL (520 nmol/L) | 20–29 mg/dL (520–750 nmol/L) | ≥ 30 mg/dL (780 nmol/L) |
How Remnant Cholesterol Contributes to Cardiovascular Disease
Remnant cholesterol contributes to atherosclerotic cardiovascular disease through several pathways, including direct cholesterol deposition, activation of inflammation, and vascular dysfunction.53 Like LDL particles, remnant lipoproteins can penetrate the arterial wall and deposit cholesterol beneath the endothelium.53 However, it is estimated that a single remnant particle may carry up to 40 times more cholesterol than each LDL particle, amplifying its ability to promote foam cell formation, an early hallmark of atherosclerosis.36 Furthermore, while LDL typically requires oxidative modification before being taken up by macrophages, remnant particles can be absorbed more readily through alternative cellular pathways.54,55
Beyond direct cholesterol deposition, remnant particles contribute to vascular injury by promoting inflammation and endothelial dysfunction. As remnant particles undergo partial breakdown and metabolism, they release free fatty acids and other byproducts that reduce nitric oxide availability and increase oxidative stress within the blood vessel wall.55,56 These effects contribute to endothelial dysfunction and stimulate the release of inflammatory cytokines, often reflected by elevated levels of C-reactive protein (CRP).12,57 In addition, remnant cholesterol is associated with increased production of prothrombotic factors and the upregulation of adhesion molecules, creating a vascular environment that promotes clot formation and atherosclerosis.21,58 Collectively, these mechanisms illustrate how remnant cholesterol contributes to an inflamed and dysfunctional vascular environment, particularly in those with insulin resistance and metabolic dysfunction.
Why Remnant Cholesterol May Matter More Than LDL-C
LDL-C is a well-established and modifiable risk factor of cardiovascular disease. However, a growing body of evidence suggests that remnant cholesterol may play an equally important, and in some cases stronger, role in the development of atherosclerosis than LDL-C.7,13 This article does not seek to diminish the importance of LDL-C, but rather to examine emerging evidence supporting the independent and causal role of remnant cholesterol in atherosclerosis, even when LDL-C and Apolipoprotein B (ApoB) levels are within optimal ranges.59 Despite its significance, remnant cholesterol remains underrecognized and is less responsive to traditional lipid-lowering therapies aimed at reducing LDL-C and ApoB.14,15,16,17,21-23
Observational Studies
Multiple prospective studies have demonstrated that remnant cholesterol is strongly associated with future cardiovascular events and often outperforms LDL-C in predicting risk.7-9,59In one study examining more than 17,000 individuals without preexisting cardiovascular disease, remnant cholesterol emerged as a stronger predictor of incident cardiovascular events than LDL-C.8 After adjusting for traditional risk factors—including age, sex, race, smoking status, blood pressure, and lipid-lowering therapy—the hazard ratio for remnant cholesterol was 1.71, compared to 1.32 for LDL-C. When further adjusting for ApoB, the association between LDL-C and cardiovascular events was no longer statistically significant, whereas remnant cholesterol remained predictive with a hazard ratio of 1.65. These findings suggest that remnant cholesterol captures residual cardiovascular risk not explained by LDL-C or ApoB alone. While LDL-C remains clinically relevant, its contribution to cardiovascular risk appears to be fully accounted for by ApoB, whereas remnant cholesterol confers risk independently of ApoB levels.
Longitudinal Coronary Artery Calcium Imaging
Beyond clinical outcomes, remnant cholesterol has also been associated with the progression of atherosclerosis identified on cardiovascular imaging studies.10 In a combined analysis of participants from the CARDIA and MESA studies, researchers observed that higher baseline remnant cholesterol predicted more rapid progression of coronary artery calcium (CAC) over a 10-year period. Even after adjusting for LDL-C and other traditional risk factors, each 1 mg/dL increase in remnant cholesterol was associated with a 1.3% increased risk of CAC progression. Notably, individuals with high remnant cholesterol but low LDL-C experienced significantly greater calcium accumulation compared to those with low levels of both. These findings suggest that remnant cholesterol may contribute to subclinical plaque development even when LDL-C is controlled.
Mendelian Randomization
Mendelian randomization, which uses genetic variants to explore causal relationships between biomarkers and disease, has provided strong evidence for the independent role of remnant cholesterol in cardiovascular risk. In one study using UK Biobank data, researchers compared the effects of remnant cholesterol and LDL-C on coronary heart disease risk.59 The odds ratio per 1 mmol/L increase in remnant cholesterol was 2.59, compared to 1.37 for LDL-C, suggesting that remnant particles are substantially more atherogenic per unit increase.
Another Mendelian randomization study extended these findings to a broader range of cardiovascular illnesses.11 In this analysis, remnant cholesterol was associated with significantly increased odds of coronary artery disease (OR 1.51), heart attack (OR 1.57), and stroke (OR 1.23) per standard deviation increase. In contrast, LDL-C demonstrated lower odds ratios for these outcomes (1.26, 1.18, and 1.14, respectively). Importantly, the association between remnant cholesterol and coronary events remained significant after adjusting for LDL-C and ApoB, suggesting a distinct, causal mechanism of risk.
Additional evidence links remnant cholesterol to both coronary artery disease and systemic inflammation. In a separate study, a 1 mmol/L increase in remnant cholesterol was associated with a 3.3-fold higher risk of coronary artery disease, compared to a 1.8-fold risk associated with LDL-C.12 Remnant cholesterol was also linked to a 28% increase in C-reactive protein (CRP) levels, whereas LDL-C showed no meaningful effect on systemic inflammation. These associations were consistent across individuals with and without diabetes or obesity.
Finally, a recent Mendelian randomization analysis involving over 38,000 cases of peripheral artery disease and 221,000 cases of coronary artery disease further confirmed the risk associated with remnant cholesterol.13 A 1 mmol/L increase in remnant cholesterol was associated with a 2.16-fold higher risk of peripheral artery disease, compared to a nonsignificant 1.14-fold increase for LDL-C. Notably, remnant cholesterol remained significantly associated with coronary artery disease risk even after accounting for LDL-C and ApoB levels.
Concluding Remarks
Both LDL-C and remnant cholesterol contribute causally to atherosclerotic cardiovascular disease, with remnant cholesterol emerging as an independent risk factor even when LDL-C and ApoB levels are optimal.7,8,13,21,24 These findings highlight the importance of evaluating triglyceride-rich lipoproteins, particularly in individuals with metabolic syndrome, diabetes, or hypertriglyceridemia, where remnant cholesterol may drive substantial residual risk.
At the same time, ApoB remains the most comprehensive marker of atherogenic particle burden and should continue to be prioritized for risk stratification. It is also important to recognize that LDL particles are far more abundant than remnant particles, and that therapeutic efforts to lower LDL-C have been more successful than efforts to lower triglyceride-rich lipoproteins. Rather than viewing one risk factor as more important than the other, an optimal strategy for promoting cardiovascular health and longevity should aim for favorable levels of both remnant cholesterol and ApoB.
The Resistance of Remnant Cholesterol to Statin Therapy and Saturated Fat Restriction
Recognition of LDL and ApoB as causal risk factors for cardiovascular disease has led healthcare guidelines to emphasize the optimization of these levels through dietary modification and prescription medications. Standard recommendations typically include dietary restriction of saturated fat, increased fiber intake, and statin therapy when clinically appropriate. However, due to differences in the metabolic pathways affecting LDL and remnant cholesterol, remnant cholesterol appears relatively resistant to these interventions. As a result, individuals with elevated remnant cholesterol often require targeted strategies that differ from traditional LDL-lowering approaches. In fact, some efforts to reduce LDL-C, such as replacing saturated fat with refined carbohydrates, may inadvertently increase remnant cholesterol.16
Saturated Fat Restriction
Remnant cholesterol, which is rich in triglycerides, is less favorably influenced by saturated fat restriction and is more strongly affected by carbohydrate and sugar intake.14-17 It is also important to recognize that the metabolic response to dietary interventions varies among individuals, and that pre-existing metabolic health status modifies the response to saturated fat intake. For example, high saturated fat intake may increase very low-density lipoprotein (VLDL) production, and thus remnant cholesterol, in some individuals, particularly when combined with a high-carbohydrate diet.60 However, in most cases, carbohydrate restriction exerts a more consistent and direct effect in lowering triglyceride-rich lipoproteins and remnant cholesterol.
To demonstrate this point, a randomized controlled trial assessed the effects of a high saturated fat diet compared to a low saturated fat diet in individuals with abnormal lipoprotein profiles.61 Carbohydrate intake remained similar between groups, accounting for approximately 37 to 39% of total energy intake, with the primary adjustment involving unsaturated fat consumption. Although the high saturated fat diet led to increased ApoB and LDL-C levels, there was no appreciable impact on VLDL and IDL levels, the main precursors of remnant cholesterol.
In a second randomized trial, researchers evaluated the effects of varying carbohydrate and saturated fat intake on lipoprotein profiles.14 Participants were assigned to diets containing either 8% or 15% saturated fat, with carbohydrate intake ranging from 54% to 26% of total energy. Carbohydrate restriction to 26% of energy intake led to the most significant reductions in remnant cholesterol and ApoB, independent of weight loss. In contrast, increasing saturated fat intake from 8% to 15% in the context of low-carbohydrate consumption had minimal impact on remnant cholesterol. While saturated fat may increase ApoB-containing lipoproteins such as LDL, it does not strongly influence VLDL or IDL levels when carbohydrate intake is moderate.
Statin Therapy
Although statins are highly effective at lowering LDL-C and ApoB, their impact on remnant cholesterol is considerably less pronounced. Depending on dose and potency, clinical trials have demonstrated LDL-C reductions of 30% to 50% with statin therapy, whereas reductions in remnant cholesterol typically range from 15% to 25%.21 For example, in the JUPITER trial, Rosuvastatin 20 mg daily reduced LDL-C by 50% but lowered remnant cholesterol by only approximately 20%.22 Similarly, a broader review of clinical trials found that statins reduced LDL-C by 35% to 40%, whereas remnant cholesterol was reduced by only 15% to 25%.23
These findings highlight that the primary mechanism of action for statin therapy, the upregulation of LDL receptors for increased ApoB clearance, is less effective at clearing triglyceride-rich lipoproteins, which are larger and utilize alternative clearance pathways. This relative resistance is even more pronounced in individuals with elevated baseline triglycerides, metabolic syndrome, or type 2 diabetes, where remnant cholesterol often remains elevated despite optimal LDL-C control according to conventional guidelines.21
How Sugars and Processed Carbohydrates Elevate Remnant Cholesterol
High intake of processed carbohydrates, refined grains, and foods with added sugar promotes the overproduction of very low-density lipoproteins (VLDL) in the liver, leading to elevated circulating triglycerides and an increase in remnant cholesterol levels.14,16,17,18,29 This effect has been observed in both healthy individuals and those with underlying metabolic dysfunction.18 Meanwhile, carbohydrate quality remains an important consideration, as rapidly absorbed sugars and high-glycemic-load carbohydrates contribute more substantially to increases in remnant cholesterol.18,52
Mechanistically, simple sugars, especially fructose and sucrose, stimulate hepatic de novo lipogenesis, resulting in excess synthesis and secretion of triglyceride-rich VLDL particles.37 Unlike glucose, fructose is metabolized rapidly and preferentially by the liver, bypassing key regulatory steps that ordinarily limit lipid synthesis.38 This unique metabolic pathway substantially amplifies VLDL production and contributes directly to remnant cholesterol accumulation.39
Experimental studies support these mechanistic findings. In a randomized crossover trial, 16 overweight adults consumed 25% of their total energy intake from either fructose or complex carbohydrates for nine days, separated by a 10-day washout period.18 Total energy intake was kept constant, ensuring an isocaloric comparison. Fructose consumption doubled hepatic de novo lipogenesis, increased triglycerides by 20% to 30%, and elevated VLDL levels compared to the complex carbohydrate diet. Remnant cholesterol, estimated from changes in non-HDL cholesterol and triglycerides, was also significantly higher following the fructose-rich diet. Additional trials have consistently demonstrated similar findings.37,40-42
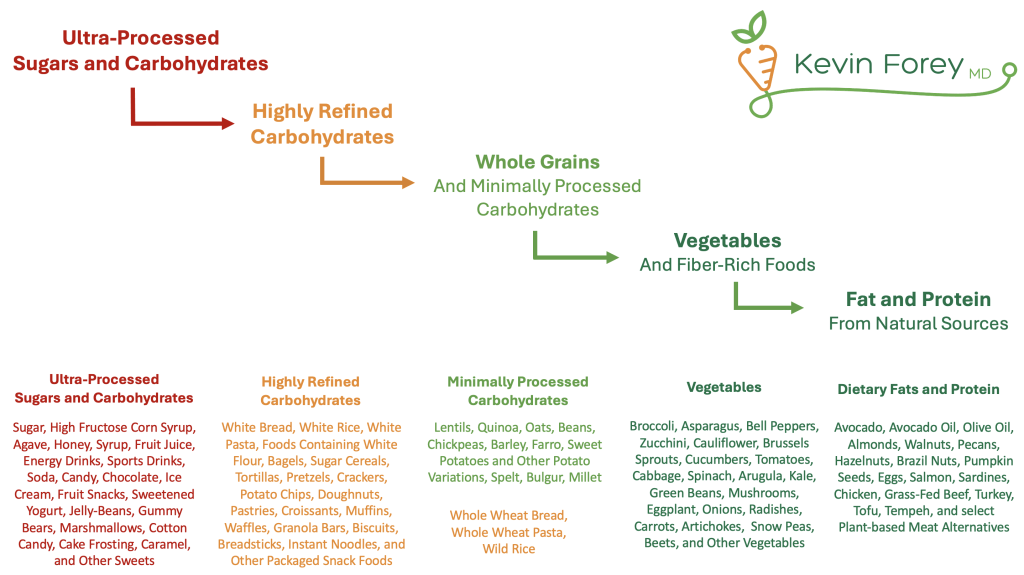
Figure 2. Stepwise Approach to Improving Carbohydrate Quality
Strategies to Lower Remnant Cholesterol
The following strategies aim to lower remnant cholesterol by targeting key mechanistic pathways, including hepatic lipogenesis, VLDL overproduction, and impaired lipoprotein clearance.
- Reduce intake of processed carbohydrates and foods with added sugar
Minimizing or eliminating the consumption of processed carbohydrates and added sugars reduces hepatic de novo lipogenesis and VLDL production. Sucrose, fructose and high-glycemic-load diets have been shown to increase VLDL levels and postprandial triglycerides.18,37,38,41,42 - Improve the quality of carbohydrate consumed
Although traditional Mediterranean diets can reduce levels of remnant cholesterol, combining Mediterranean dietary patterns with low-glycemic carbohydrate principles yields greater improvements.51 These findings reinforce that carbohydrate quality meaningfully influences metabolic health, independent of total caloric or carbohydrate intake.18,52 - Minimize or eliminate alcohol consumption
A reduction in alcohol intake can have a meaningful impact on remnant cholesterol, as alcohol increases hepatic triglyceride synthesis and VLDL secretion.45,46 - Avoid overfeeding, especially high-fat, high-carbohydrate combinations
Excess caloric intake, particularly from diets high in both fat and carbohydrates, drives hepatic lipogenesis and remnant cholesterol accumulation. Short-term overfeeding studies have demonstrated meaningful increases in remnant cholesterol and triglycerides,43 even among healthy individuals.44 - Achieve and maintain a healthy weight
Weight loss improves insulin sensitivity, reduces hepatic fat accumulation, and decreases VLDL overproduction. Clinical studies consistently demonstrate that weight loss reduces triglycerides and remnant cholesterol.49,50 - Maximize physical fitness and cardiorespiratory fitness
Regular aerobic exercise enhances lipoprotein lipase activity, promoting more efficient clearance of triglyceride-rich lipoproteins. Higher volumes of aerobic exercise, even without weight loss, improve lipoprotein clearance and favorably modify the lipoprotein profile. Although total exercise volume appears more influential than intensity alone, combining higher amounts and greater intensity yields the most pronounced benefits.47,48
The Relationship of Remnant Cholesterol, Insulin Resistance, and Non-Cardiovascular Illness
Insulin resistance and remnant cholesterol are strongly associated through a bidirectional relationship. In states of insulin resistance, hepatic production of triglyceride-rich lipoproteins such as very low-density lipoproteins (VLDL) increases. These particles are subsequently metabolized into cholesterol-rich remnant particles, which are inefficiently cleared from circulation. As a result, remnant cholesterol levels often remain elevated even when LDL cholesterol appears well controlled.
Large-scale studies have demonstrated that higher levels of insulin resistance measured by HOMA-IR correlate with higher levels of remnant cholesterol levels.62 Furthermore, Mendelian randomization analyses suggest that genetically elevated remnant cholesterol may contribute to the development of insulin resistance and type 2 diabetes.63 Beyond metabolic disease, emerging evidence has demonstrated an association of remnant cholesterol and Alzheimer’s disease,25,26 vascular dementia,25,27 certain cancers,30-32 chronic inflammation,12 and autoimmune diseases.33-35
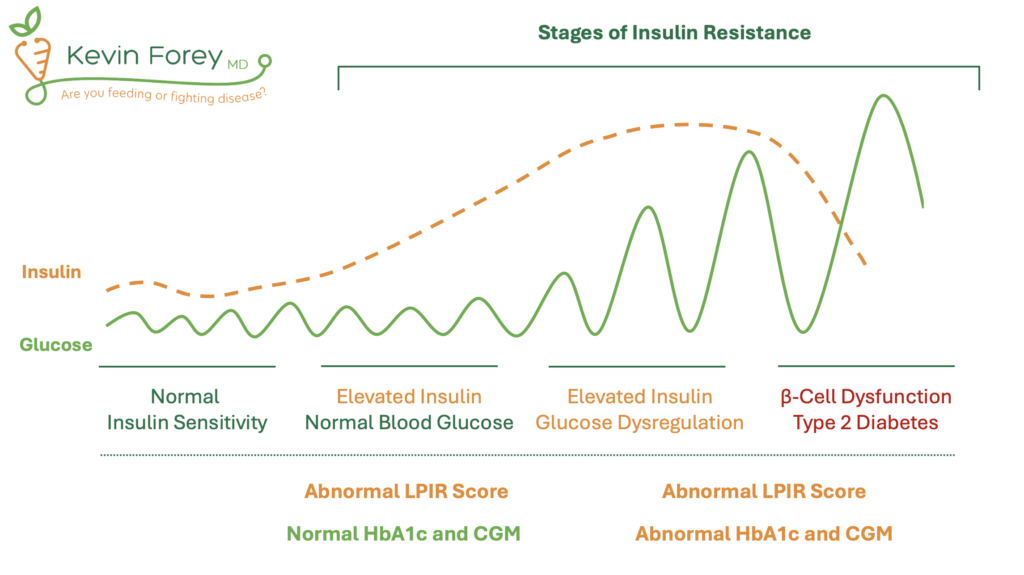
These associations underscore the importance of early detection of both insulin resistance and elevated levels of remnant cholesterol, ideally before overt clinical disease develops. Although commonly used, Hemoglobin A1c (HbA1c) is not an optimal test for identifying early stages of insulin resistance (Figure 3) HbA1c reflects average blood glucose over several months but lacks sensitivity for detecting metabolic dysfunction in individuals who maintain normal glucose levels.64,65 As a result, reliance on HbA1c alone often allows insulin resistance to progress undetected until more advanced disease emerges. Similarly, Continuous Glucose Monitors (CGMs), although useful for identifying glucose fluctuations, may fail to detect insulin resistance in individuals who maintain euglycemia through compensatory hyperinsulinemia. Therefore, an optimal strategy is to utilize superior tests that identify insulin resistance prior to the onset of blood glucose dysregulation. This includes the LPIR Score, Triglyceride-Glucose Index (TyG Index), and HOMA-IR.
Table 2. Early and Accurate Identification of Insulin Resistance
| No Insulin Resistance | Elevated Glucose Possibly Due to Reasons Other Than Insulin Resistance | Early Insulin Resistance | Early to Advanced Insulin Resistance +/- Diabetes |
| Normal HbA1c | Slightly Elevated HbA1c | Normal HbA1c | Abnormal HbA1c |
| Normal LPIR Score, TyG Index, and HOMA-IR | Normal LPIR Score, TyG Index, and HOMA-IR | Abnormal LPIR Score, TyG Index, and/or HOMA-IR | Abnormal LPIR Score, TyG Index, and/or HOMA-IR |
Figure 3. Stages of Insulin Resistance
How Insulin Resistance Increases the Atherogenicity of LDL-C and Other ApoB Particles
Insulin resistance increases the atherogenicity of ApoB-containing lipoproteins through several distinct mechanisms, including increased production, impaired clearance, prolonged circulation time, and structural modifications that promote arterial penetration and oxidative susceptibility. Studies using leucine tracer infusions have demonstrated that insulin resistance upregulates hepatic secretion of ApoB-containing particles, particularly in the form of VLDL, thereby increasing the overall burden of circulating atherogenic lipoproteins.66 Experimental evidence also shows that insulin resistance impairs the clearance of ApoB-containing particles, resulting in prolonged exposure of the arterial wall to these atherogenic lipoproteins.67 In addition, insulin resistance has been associated with enhanced oxidative modification of LDL and Lipoprotein(a) particles, further amplifying their capacity to promote atherosclerosis.29,68,69 Therefore, even among individuals who achieve guideline-recommended targets for LDL-C and ApoB, optimizing metabolic health and addressing insulin resistance remain essential strategies for those seeking to promote longevity and further reduce cardiovascular risk.