The Toxic Food Hypothesis: Rethinking Chronic Disease, Nutrition, and Calorie Balance
Introduction
Despite decades of dietary advice to “eat less and exercise more,” rates of chronic disease in America continue to rise. In an effort to explore the underlying causes of this trend, Derek Thompson, host of the Plain English podcast, released an episode titled “A Grand, Unified Theory of Why Americans Are So Unhealthy.” In this episode, he featured two respected physicians, Dr. David Kessler and Dr. Eric Topol, who presented a compelling explanation of America’s increasing rates of chronic disease and poor health outcomes. At its core, Thompson’s theory highlights a mismatch between our evolutionary biology and today’s food environment, heavily composed of ultra-processed, calorie-dense foods. This overconsumption of calories, they argue, contributes to visceral fat accumulation, chronic inflammation, and a cascade of illnesses including type 2 diabetes, cardiovascular disease, cancer, and dementia.
While Thompson’s framework is logical and biologically plausible, it leans heavily on a calorie-centric, weight-focused model of disease that has dominated public health messaging for decades. Notably, Thompson himself acknowledges that the long-standing advice to “eat less and exercise more” has coincided with worsening health outcomes across all age groups. Rather than questioning the scientific validity of this theory, the episode shifts attention to GLP-1 receptor agonists as a promising solution to a problem that we cannot otherwise solve. Importantly, Thompson closes the episode by framing his theory not as settled science, but as a working hypothesis. In that same spirit, this article introduces an alternative framework, The Toxic Food Hypothesis, that emphasizes the importance of food quality and metabolic health, while addressing several key limitations of Thompson’s model.
Importantly, excess body weight alone does not fully capture disease risk, as many individuals with cardiovascular disease, cancer, or dementia have a normal body weight.1-4 Furthermore, when compared to other metabolic risk factors such as insulin resistance and high blood pressure, body weight appears to be a relatively weak predictor of cardiovascular disease.5 Instead, a framework that more comprehensively captures metabolic dysfunction would identify tens of millions of additional at-risk individuals. The most significant shortcoming of Thompson’s grand unified theory is the lack of evidence supporting its premise. Randomized clinical trials have not shown that calorie restriction reduces cardiovascular disease or all-cause mortality, calling into question its central role in disease prevention.6-8 In contrast, other landmark dietary trials have achieved significant reductions in cardiovascular death and disease by improving calorie quality without reducing total intake.9 These findings highlight the need for an alternative framework that accounts for a wider array of metabolic risk factors and their underlying causes.
This alternative framework, termed The Toxic Food Hypothesis, shifts focus away from calorie counting and body weight toward a broader view of metabolic health, recognizing that chronic disease affects those with excess weight as well as millions of lean individuals who share similar metabolic risk factors. At its core, the hypothesis argues that the primary cause of America’s declining health is not simply the number of calories consumed, but the widespread displacement of whole foods with highly processed food products. While there is agreement that these foods are calorie-dense and promote overeating,10,39 a growing body of evidence suggests that highly processed foods negatively impact health through multiple mechanisms independent of calorie intake.11-15 Furthermore, clinical trials have demonstrated that reducing or eliminating processed foods, even without reducing calorie intake, can meaningfully improve insulin sensitivity, blood pressure, inflammation, and body composition.11
The Toxic Food Hypothesis does not dismiss the harms of excess calorie intake but reframes overconsumption as a secondary consequence of a distorted food environment rather than a primary failure of individual willpower. Public health messaging that continues to emphasize “eat less and exercise more” misses the deeper structural problem of a food system dominated by highly processed foods.16 Instead of encouraging Americans to “eat less, exercise more, and consider a GLP-1 agonist,” public health guidelines should explicitly discourage the routine consumption of highly processed foods, much as we do with tobacco, indoor tanning, and other avoidable health hazards. Furthermore, physical fitness should be promoted due to its numerous, independent health benefits rather than as a tool to offset dietary choices within a calorie-balance framework.
Interestingly, the most frequent criticism of this perspective focuses not on the science, but on the difficulty of defining “ultra-processed foods.” While valid, this critique overlooks the complexity of today’s food system and human biology. With tens of thousands of unique items in a typical grocery store, expecting a single, universally applicable definition is neither practical nor necessary. Much like health and disease, which exist on a spectrum rather than as black-and-white categories, the food landscape is similarly nuanced. Although the term “ultra-processed” lacks precise definition and must allow for exceptions, this should not distract from the growing body of evidence linking these foods to adverse health outcomes, driven by several distinct mechanisms.
Taken together, these insights justify a shift in focus from calorie counting and body weight to a broader understanding of food quality and metabolic health. The Toxic Food Hypothesis presents a more comprehensive, inclusive, and evidence-based framework that accounts for rising rates of illness in both lean and overweight populations. Furthermore, it also challenges the reader to question whether the energy-balance narrative truly reflects the full weight of scientific evidence or is simply accepted because of repetition and familiarity. Progress in nutritional science begins not with certainty, but with the willingness to question what we think we know.
Related Podcast Episode
Disclaimer
This content is for general educational purposes only and does not represent medical advice or the practice of medicine. Furthermore, no patient relationship is formed. Please discuss with your personal healthcare professional before making any dietary, lifestyle, or medication changes. Additionally, I have no financial conflicts of interest or affiliations with any diagnostic testing or pharmaceutical companies mentioned in this article.
Notify Me of New Content
Provide your email address to receive notifications of new blog posts and podcast episodes.
Content Summary
Obesity Represents a Narrow Focus of Risk
Obesity is often portrayed as the central cause of chronic illness, yet it captures only a portion of those at risk. Millions of individuals with a normal body weight still develop cardiovascular disease, dementia, cancer, and type 2 diabetes, often due to underlying metabolic abnormalities.1-4 While roughly 70% of U.S. adults are overweight or obese, approximately 90% show at least one sign of metabolic dysfunction, including insulin resistance, elevated blood pressure, abnormal cholesterol levels, or excess abdominal fat, all of which are independent risk factors of chronic illness.17-20 Among adults with a normal body weight, fewer than one-third are metabolically healthy, and roughly 16% of adolescents with a normal body weight exhibit signs of insulin resistance.20,26 These findings underscore the need for a framework that prioritizes metabolic health, and in turn, may provide more effective solutions than calorie restriction alone.
Obesity Is a Relatively Weak Predictor of Disease
When compared to other modifiable risk factors, obesity appears to be a weaker predictor of cardiovascular disease than insulin resistance, high blood pressure, and abnormal cholesterol patterns.5 To demonstrate this point, the Women’s Health Study followed more than 28,000 women over 21 years and compared a variety of cardiovascular risk factors (Table 1).5 Among women who developed cardiovascular disease before age 55, obesity was associated with a 4.3-fold increase in risk (Hazard Ratio 4.3). While substantial, it is far lower than the risk attributed to diabetes (HR 10.7) or metabolic syndrome (HR 6.1), and slightly lower than elevated blood pressure (HR 4.6). By ages 65 to 75, the relationship between obesity and cardiovascular disease weakened further (HR 1.3), while diabetes, metabolic syndrome, and elevated blood pressure continued to predict risk more strongly.
When risk factors were measured as continuous variables, insulin resistance, measured by the LPIR score, was four times more predictive than BMI for early-onset cardiovascular disease (HR 6.4 vs. 1.5). Triglycerides, systolic blood pressure, Apolipoprotein B (ApoB), and C-Reactive Protein (CRP), also consistently outperformed BMI as risk factors of cardiovascular disease (Table 2). Importantly, these metabolic abnormalities confer independent cardiovascular risk, underscoring that metabolic dysfunction is a separate, powerful driver of chronic disease, and not simply a consequence of excess body weight.
Table 1. Comparative Risk of Traditional Cardiovascular Risk Factors by Age at Disease Onset5
| Age of Onset < 55 Years Adjusted Hazard Ratio | Age of Onset 65 – 75 Years Adjusted Hazard Ratio | |
| Diabetes | 10.71 | 3.47 |
| Metabolic Syndrome | 6.09 | 1.79 |
| Hypertension | 4.58 | 1.64 |
| Obesity, BMI > 30 kg/m2 | 4.33 | 1.32 |
Table 2. Risk Factors of Cardiovascular Disease by Age of Onset in the Women’s Health Study5
| Risk Factor, per SD Increment | Age of Onset < 55 Years Adjusted Hazard Ratio | Age of Onset 65 – 75 Years Adjusted Hazard Ratio |
| Insulin Resistance, LPIR Score | 6.40 | 2.09 |
| Systolic Blood Pressure | 2.24 | 1.48 |
| Triglycerides | 2.14 | 1.61 |
| Apolipoprotein B (ApoB) | 1.89 | 1.52 |
| C-Reactive Protein (CRP) | 1.76 | 1.62 |
| Body Mass Index (BMI) | 1.47 | 1.33 |
| LDL Cholesterol (LDL-C) | 1.38 | 1.24 |
Reassessing Calorie Restriction: Limited Evidence of Mortality or Cardiovascular Benefit
One of the most significant challenges to the energy-balance model of disease is the lack of clinical evidence supporting its effectiveness in preventing chronic illness and prolonging life. Despite decades of public health messaging urging Americans to “eat fewer calories,” major trials have failed to demonstrate that calorie restriction meaningfully reduces cardiovascular disease, cancer, or mortality.6-8
Two landmark studies, the Multiple Risk Factor Intervention Trial (MRFIT) and the Women’s Health Initiative Dietary Modification Trial (WHI-DM), tested strategies consistent with traditional dietary advice, including lowering fat intake, increasing fruits, vegetables, and whole grains, and modestly reducing total calorie intake.7,8 While neither trial directly assessed the effect of calorie restriction, both achieved modest reductions in calorie intake compared to control groups. Despite these efforts, neither study demonstrated reductions in heart disease, cancer incidence, or mortality.7,8
In a separate study, the Look AHEAD trial sought to provide a more direct evaluation of the effect of calorie restriction and health outcomes.6 This study enrolled more than 5,000 overweight and obese adults with type 2 diabetes, instructing the intervention group to reduce calorie intake by 25 to 30%, aiming for at least a 7% weight loss at one year. Participants surpassed this goal, losing an average of 8.6% of body weight at one year and maintaining roughly 6% weight loss at four years. Despite nearly a decade of calorie restriction and sustained weight loss, the trial failed to reduce cardiovascular events compared to standard care.6
In contrast, the PREDIMED trial demonstrated that substantial improvements in major adverse cardiovascular events can be achieved without calorie restriction.9 This study enrolled more than 7,400 high-risk individuals to evaluate two variations of the Mediterranean diet, one enriched with extra-virgin olive oil and the other with mixed nuts. Participants were not instructed to reduce calories, yet both groups achieved a roughly 30% reduction in heart attack, stroke, and death from cardiovascular disease. These benefits occurred alongside a modest weight loss of 2.2 pounds (1 kg), suggesting that these improvements were largely independent of body weight. Additionally, a secondary analysis revealed a 52% reduction in new-onset type 2 diabetes among participants without baseline diabetes.27
Although the definition of the Mediterranean diet is interpreted in many ways, several features of the PREDIMED intervention help explain its impact. In both variations of the diet, carbohydrate intake was reduced as participants increased dietary fat intake from naturally occurring food sources, such as olive oil and mixed nuts. Among the remaining carbohydrates consumed, participants were instructed to replace highly refined carbohydrates with minimally processed carbohydrates, such as whole grains and legumes. These dietary adjustments likely improved insulin resistance and other markers of metabolic dysfunction, helping explain why both groups achieved large reductions in heart attack, stroke, and cardiovascular death despite no significant weight loss or calorie reduction. While some cite the presumed benefits of antioxidants within olive oil, similar benefits observed in the mixed nut group suggest that the broader dietary adjustments were responsible for the observed health benefits, rather than a single nutrient. The results of PREDIMED underscore that the quality of calories consumed deserve greater consideration as a strategy to prevent chronic disease.
Food Quality Can Impact Metabolic Health Independent of Calorie Intake
Metabolic health can improve even without reducing total calorie intake.11 In one study, researchers enrolled over 40 overweight children and lowered their sugar intake from 28% to 10% of total calories, replacing the sugar with starch-based carbohydrates to keep total calorie intake and carbohydrate intake unchanged.11 Within 10 days, fasting insulin was reduced by 53% and triglycerides by 46%, with additional improvements seen in blood pressure, inflammation, and liver dysfunction. Similar benefits have been demonstrated in adults. In the A TO Z Weight Loss Study, it was demonstrated that the greatest reduction in carbohydrates and simple sugars resulted in the greatest reductions in blood pressure, body weight, and blood glucose control, even when calorie intake remained constant.28
While highly processed foods have been shown to contribute to overconsumption, there are multiple calorie-independent mechanisms by which processed foods contribute to metabolic dysfunction and inflammation. Refined sugars, particularly fructose, are strongly implicated in insulin resistance and visceral adiposity.11,12,29 Meanwhile, industrially manufactured trans fats and hydrogenated oils have been shown to elevate inflammatory markers and disrupt cholesterol profiles, regardless of total energy intake.13-15
Why Defining Highly Processed Foods Matters, Even If Imperfect
A common critique of the Toxic Food Hypothesis is the difficulty of defining what qualifies as “highly processed foods.” With more than 30,000 distinct food products in a typical grocery store, a single, rigid definition is neither practical nor realistic. For this discussion, highly processed foods refer to food products that cannot be made at home using simple ingredients and basic food preparation techniques. Importantly, cooking is not the same as industrial food processing. Unlike household food preparation (e.g., chopping vegetables, heating food, grinding peanuts into peanut butter), industrial processing involves chemical modifications, additives, and ingredients such as emulsifiers, synthetic flavorings, and stabilizers that fundamentally alter a food’s nutritional profile, function, and composition. A useful approach is to evaluate food qualitatively along a spectrum:
Naturally Occurring → Minimally Processed → Highly Processed → Ultra-Processed
Not all processed foods are inherently harmful, and exceptions to this framework exist. For example, traditional plant-based foods like tofu and tempeh are minimally processed, contain few ingredients, and are associated with favorable or neutral metabolic effects.30-33 Similar findings have been observed in trials evaluating the metabolic impact of dairy products.34-37 A common characteristic of these exceptions is that they are produced from a small number of whole-food ingredients, typically of plant or animal origin, rather than from refined grains, flours, manufactured oils, or chemically modified components. Meanwhile, the overwhelming majority of foods contributing to poor health are highly processed and manufactured in industrial settings. These products account for roughly 90% of added sugar, 70% of dietary sodium, and most trans fats and hydrogenated oils in the American diet.21-23 Rather than simply telling people to “eat less sugar and salt,” a more effective public health message is to minimize or eliminate the regular consumption of highly processed foods.
The Toxic Food Hypothesis
Over the past century, the global food system has been reshaped by technological advances, shifting household dynamics, changes in the workforce, and a growing preference for convenience over quality. As a result, highly processed foods account for nearly 60% of total calorie intake in America.16 Meanwhile, rates of obesity, insulin resistance, and metabolic syndrome have risen dramatically across all age groups.38
The Toxic Food Hypothesis challenges the conventional calorie-centric model of disease, emphasizing that food quality, not just quantity, is the primary explanation for worsening health observed in America. Despite growing evidence linking highly processed foods to poor metabolic health, mainstream nutrition guidelines continue to emphasize a “balanced diet” without adequately addressing the unique harms of these foods. The common recommendation to “eat everything in moderation” fails to acknowledge that some foods are inherently detrimental to human health. Rather than moderation, a more effective approach is to minimize or eliminate the regular consumption of highly processed foods.
The Toxic Food Hypothesis identifies three primary mechanisms through which highly processed foods contribute to chronic disease, each supported by robust scientific evidence. While these pathways form the foundation of the hypothesis, other potential mechanisms are being actively studied, including gut microbiome disruption, hormone interference from additives and packaging, and epigenetic changes.
- Overconsumption: These foods are deliberately engineered to be highly palatable, calorie-dense, and difficult to resist. Their design encourages rapid, repeated, and excessive consumption compared with whole or minimally processed alternatives.10,39
- Metabolic Dysfunction: Even when calorie intake is unchanged, highly processed foods negatively impact insulin sensitivity, blood pressure, cholesterol levels, and the development of fatty liver disease.11
- Inflammation: Similarly, even when calorie intake remains constant, industrially-manufactured trans fats and hydrogenated oils negatively impact inflammation and cholesterol levels.13-15
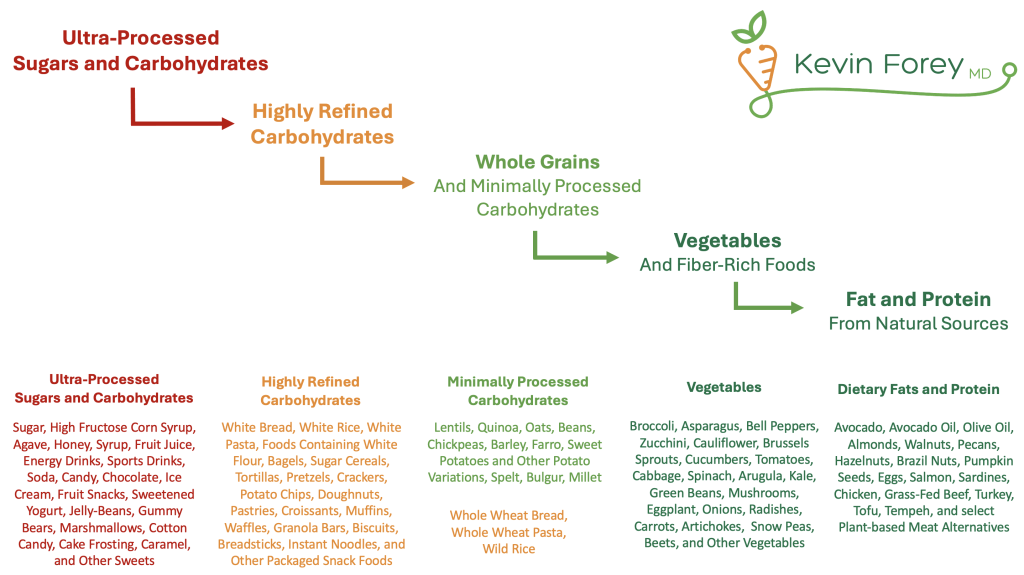
This framework emphasizes simplicity over perfection. It does not demand the rigid elimination of all packaged or processed foods but advocates for a deliberate reduction in the regular consumption of highly processed food products. For most individuals, meaningful improvements in metabolic health can be achieved by minimizing or eliminating:
- Added and refined sugars
- White flour and highly refined carbohydrates
- Deep-fried foods and hydrogenated oils
- Processed and grain-fed meats
- Alcohol, tobacco, and other harmful exposures
Figure 1. A Stepwise Approach to Improving the Quality of Calories Consumed
Measuring Comprehensive Metabolic Health: Insulin Resistance Is Not the Same as Blood Glucose Control
Comprehensive metabolic health is not adequately captured by a single test or number. Instead, it is best assessed by evaluating a variety of factors, including blood pressure, visceral fat, cholesterol levels (ApoB, Triglycerides, and HDL cholesterol), markers of inflammation, muscle mass, physical strength, bone density, and cardiorespiratory fitness. Some of these risk factors directly contribute to disease (e.g., ApoB and cardiovascular disease), while others, like HDL cholesterol, primarily reflect broader metabolic health shaped by food, nutrition, and physical activity.
Within the Toxic Food Hypothesis framework, insulin resistance is considered the most important marker of metabolic health because of its strong association with cardiovascular disease (Table 1) and its contribution to a wide spectrum of chronic illnesses (Table 3). Importantly, insulin resistance is not the same as blood glucose control. For example, the body can maintain normal glucose levels by releasing more insulin. As a result, mild to severe insulin resistance can exist years before abnormalities appear in fasting glucose, HbA1c, or continuous glucose monitoring.24,25 Further complicating matters, HbA1c can be elevated in healthy individuals without insulin resistance, such as endurance athletes (who often have higher fasting glucose due to increased gluconeogenesis), those with iron deficiency, prolonged red blood cell lifespan, or those taking certain medications.
These limitations of fasting blood glucose, HbA1c, and CGM underscore the importance of utilizing tests that more directly assess insulin resistance. Rather than utilizing a single biomarker alone, a comprehensive evaluation of insulin resistance should utilize the combination of the LPIR Score, Triglyceride-Glucose Index (TyG Index), HOMA-IR, and other assessments of metabolic health.
- Lipoprotein Insulin Resistance Score (LPIR): A blood test evaluating lipoprotein particle size and concentration, calibrated against the euglycemic hyperinsulinemic clamp (the gold standard for insulin resistance measurement). LPIR can detect early insulin resistance, even in individuals with normal weight and normal glucose levels.
- Triglyceride-Glucose Index (TyG Index): Calculated from fasting triglycerides and glucose, this simple and cost-effective index outperforms HbA1c and HOMA-IR in predicting insulin resistance. TyG is also strongly associated with all-cause mortality, frailty, dementia, and multiple cancers, including breast and colon cancer.
- HOMA-IR: Based on fasting glucose and insulin, this test is highly sensitive but prone to variability due to transient changes in fasting insulin. HOMA-IR is best used in conjunction with LPIR and TyG to provide a more complete picture of metabolic health.
Figure 2. Stages of Insulin Resistance
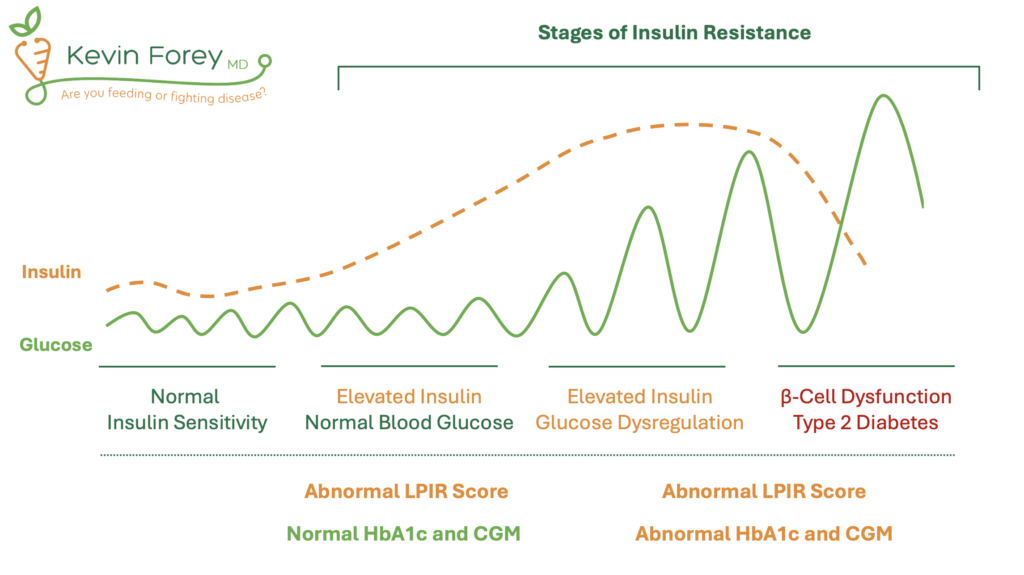
Table 3. Preventable Medical Illness Associated With Insulin Resistance
| Cancer | Colorectal Cancer | Breast Cancer | Pancreatic Cancer |
| Liver Cancer | Esophageal Cancer | Kidney Cancer | |
| Prostate Cancer | Endometrial Cancer | Ovarian Cancer | |
| Metabolic | Type 2 Diabetes | Liver Disease | Kidney Disease |
| Cardiovascular | High Blood Pressure | Heart Attack | Stroke |
| Autoimmune Disease | Ulcerative Colitis | Crohn’s Disease | Rheumatoid Arthritis |
| Inflammatory | Osteoarthritis | Gout | Acne |
| Neurological | Dementia | Parkinson’s Disease | Migraine |
| Reproductive | Erectile Dysfunction | Infertility + PCOS | Gestational Diabetes |
| Respiratory | Asthma | Sleep Apnea | Pulmonary Hypertension |
| Functional | Frailty | Muscle Loss | Osteopenia |